Search API
A new U.S. Centers for Disease Control and Prevention (CDC) report describes the use of Ebola vaccines and the role of the stockpile developed and managed by the International Coordinating Group (ICG) on Vaccine Provision.
A total of 145,690 doses have been shipped from the ICG stockpile since 2021.
Because Ebola outbreaks since 2021 have been limited, most doses (139,120; 95%) shipped from the ICG stockpile have been repurposed for preventive vaccination of high-risk groups, compared with 6,570 (5%) used for outbreak response.
The CDC wrote on April 25, 2024, repurposing doses for preventive vaccination could be prioritized in the absence of Ebola outbreaks to prevent transmission and maximize the cost-efficiency and benefits of the stockpile.
Currently, two licensed vaccines are recommended for the prevention of Ebola caused by Orthoebolavirus zairense:
- The 1-dose rVSVΔG-ZEBOV-GP (ERVEBO®) was licensed by the European Medicines Agency and the Food and Drug Administration in 2019 and is indicated for use in persons aged >12 months. The vaccine has also been approved in Burundi, Central African Republic, Côte d’Ivoire, Democratic Republic of the Congo (DRC), Ghana, Guinea, Republic of the Congo, Rwanda, Sierra Leone, Uganda, and Zambia. The U.S. FDA has approved ERVEBO.
- The 2-dose Ad26.ZEBOV and MVA-BN-Filo (Zabdeno/Mvabea) regimen is recommended for preventive vaccination in areas at lower risk for Ebola (or areas neighboring an outbreak) because the complete regimen is administered over 56 days.
To effectively manage the global Ebola stockpile, the International Coordinating Group on Vaccine Provision ensures equitable and timely access to vaccine doses for Ebola outbreaks, says the CDC.
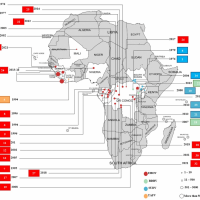
Ebolaviruses were first discovered in 1976 near the Ebola River in what is now the Democratic Republic of Congo. According to the CDC, viral and epidemiologic data suggest that ebolaviruses existed long before the initial recorded outbreaks occurred.
Soligenix, Inc. announced today that the Office of Orphan Products Development of the United States Food and Drug Administration (FDA) has granted orphan drug designation to the active ingredient in MarVax™, the subunit protein vaccine of recombinantly expressed Marburg marburgvirus (MARV) glycoprotein, for "the prevention and post-exposure prophylaxis against MARV infection."
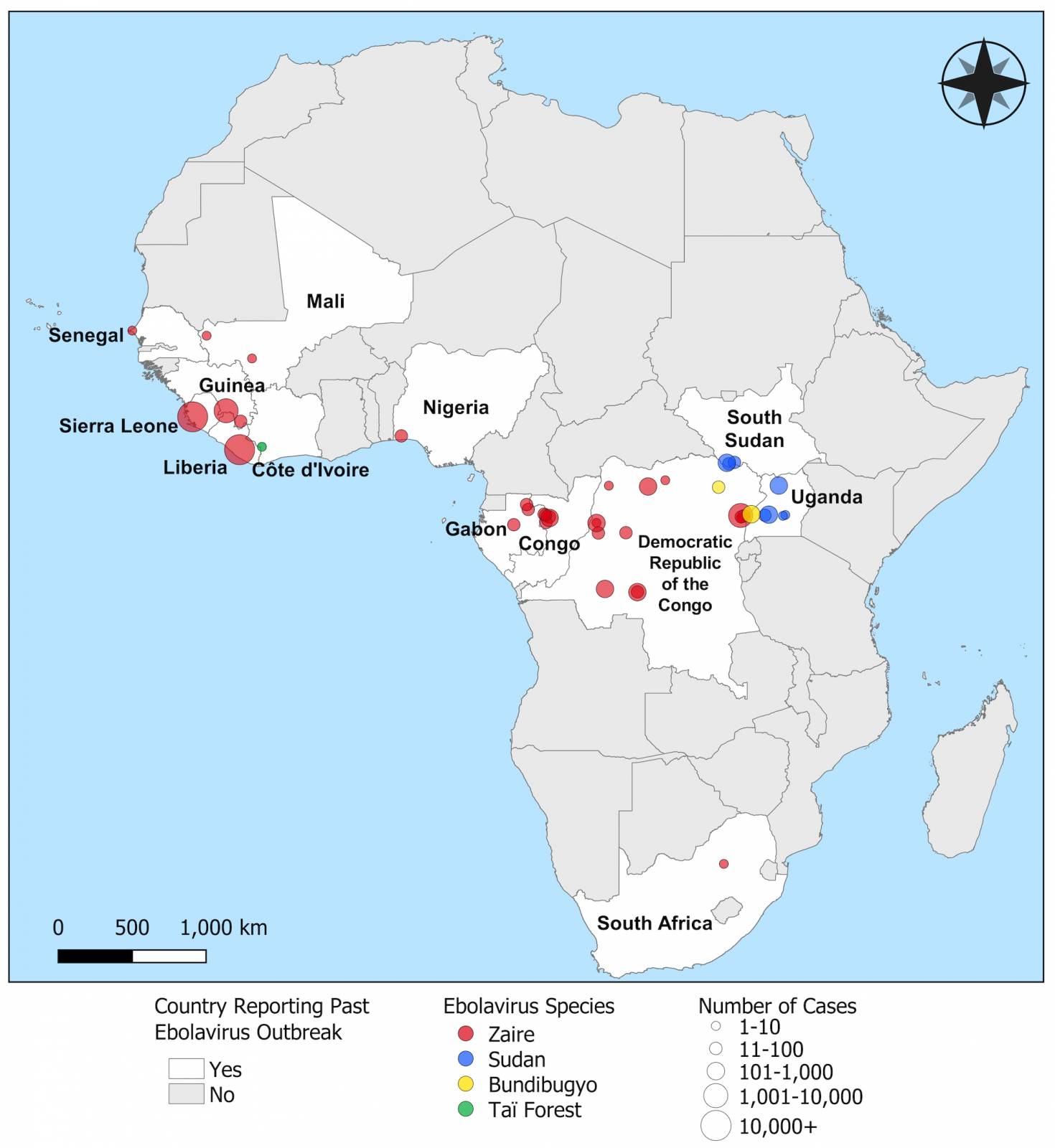
MARV is a member of the Filoviridae family, which also includes Sudan and Zaire ebolaviruses. MARV outbreaks were first recognized in 1967 In Germany and Serbia, and continue in 2024.
MarVax was developed with Dr. Axel Lehrer at the University of Hawaiʽi at Mānoa.
In addition to providing a seven-year term of market exclusivity upon final FDA approval, orphan drug designation also positions Soligenix to be able to leverage a wide range of financial and regulatory benefits, including government grants for conducting clinical trials, waiver of expensive FDA user fees for the potential submission of a Biologics License Application, and certain tax credits.
The U.S. Orphan Drug Act assists companies in developing safe and effective therapies for treating rare diseases and disorders that affect fewer than 200,000 people in the U.S.
Christopher J. Schaber, PhD, President and Chief Executive Officer of Soligenix, commented in a press release on April 15, 2024, "Elements of this subunit vaccine platform... indicate its broad applicability."
"We have also demonstrated the ability to package more than one vaccine antigen in a single vaccine, particularly against MARV and Sudan ebolavirus where there are currently no available vaccines."
"The FDA's decision to grant orphan drug designation to both the MARV and Sudan ebolavirus vaccine candidates signifies an important step for Soligenix as we continue to advance the program and adds significantly to the existing patent estate surrounding this novel technology and the filovirus program."
As of April 2024, several MARV vaccine candidates are conducting early-stage clinical studies.
An advanced vaccine candidate for the Lassa fever virus (LASV) today announced the start of its second phase of clinical trials.
This is a significant development, as no approved vaccines for LASV are currently available.
The trial sponsor, International AIDS Vaccine Initiative (IAVI), a nonprofit scientific research organization, confirmed in a press release on April 4, 2024, that participants at HJF Medical Research International in Nigeria had been vaccinated in the first Phase 2 clinical trial of the vaccine candidate rVSV∆G-LASV-GPC.
The IAVI C105/PREVAIL15 study began on March 4, 2024, and is expected to enroll over 600 people in Nigeria, Ghana, and Liberia, with results expected in 2025.
As of March 2024, 27 states in Nigeria have reported at least one confirmed case of LASV.
The vaccine has been developing since 2018 and has been supported and funded by CEPI and the European & Developing Countries Clinical Trials Partnership.
According to IAVI, rVSV∆G-LASV-GPC uses the same recombinant vesicular stomatitis virus vector platform as ERVEBO®, the single-dose Zaire ebolavirus vaccine licensed in North America, Europe, and various African countries.
“Continued outbreaks of Lassa fever and the emergence of Ebola Sudan in Uganda both underscore the need to have vaccines for known disease threats available for evaluation and use during outbreak situations – the overarching goal of IAVI’s emerging infectious disease program,” stated Swati Gupta, DrPH, MPH, vice president and head of emerging infectious diseases and epidemiology, IAVI.
The virus causes acute viral hemorrhagic illness and results in approximately 5,000 deaths and 300,000 illnesses in West Africa each year.
Furthermore, LASV has been included in the World Health Organization's R&D Blueprint of priority pathogens for which accelerated research and development and countermeasures are urgently needed.
In addition to rVSV∆G-LASV-GPC, several other LASV vaccine candidates are conducting clinical research in 2024.
Vaccines to protect people against Zaire Ebolavirus outbreaks have been used during outbreaks over the past few years.
According to the World Health Organization, two Ebola vaccines are available in 2024.
A recent study has confirmed that the prime-boost Ebola vaccine regimen is safe and effective for children and adults.
This phase 2 study assessed the long-term immunogenicity of the MVA-BN-Filo vaccine regimen and the safety of an immune memory response to an Ad26.ZEBOV booster vaccination.
These researchers concluded, in a paper published on March 26, 2024, that the vaccine regimen and booster dose were well tolerated.
These researchers wrote that a similar and robust humoral immune response was observed for participants boosted one year and two years after the first dose, supporting the use of the regimen and flexibility of booster dose administration for prophylactic vaccination in at-risk populations.
The other recommended Ebola vaccine is Merck's Ervebo®, which was approved in 2019.
However, no vaccines have been approved to protect people against the Sudan Ebolavirus.
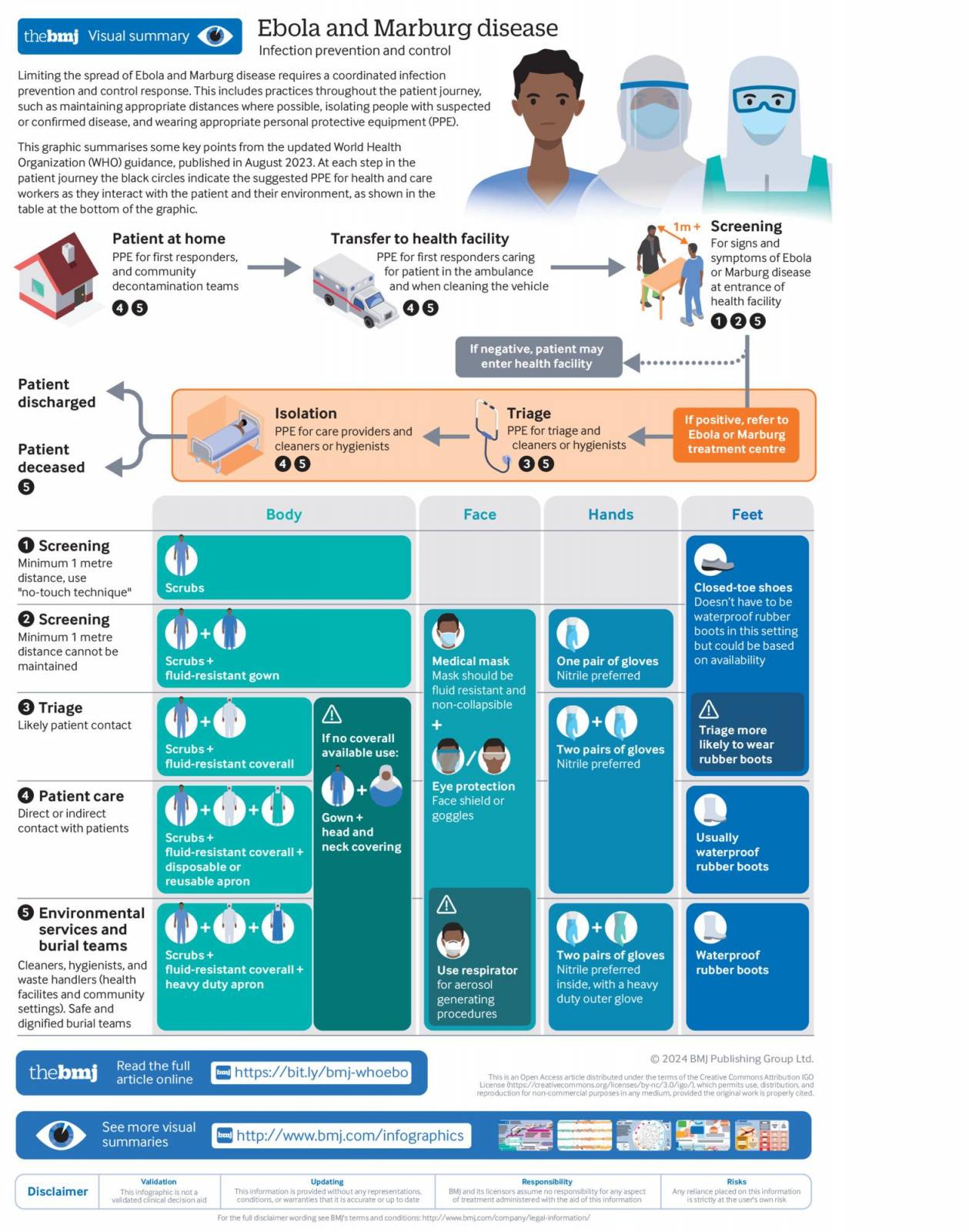
In 2024, ten years after the West African Zaire Ebola outbreak, the World Health Organization updated its guidelines on infection prevention and control for Ebola disease.

The World Health Organization (WHO) has recently updated its guidelines on infection prevention and control for Ebola disease ten years after the West African Ebola outbreak.
During outbreaks, Ebola infections have relatively high case fatality rates, averaging about 50%.
Therefore, it is crucial to have evidence-based, up-to-date infection prevention and control guidelines to ensure a safe, systematic, and standardized approach during outbreaks, said the WHO in a media release on February 27, 2024.
The complete guidelines are available on the WHO website (WHO/WPE/CRS/HCR/2023.1) and the web-based MAGICapp platform.
Since 2019, various Zaire Ebolavirus vaccines have been approved by the U.S. Food and Drug Administration, European Medicines Agency, and the WHO. These Ebola vaccines are not commercially available in 2024.
The journal Vaccine recently published a manuscript entitled "Thermostable bivalent filovirus vaccine protects against severe and lethal Sudan ebolavirus and marburgvirus infection."
This publication describes the preclinical efficacy of a novel, single-vial, bivalent thermostabilized vaccine providing 100% protection in the most rigorous non-human primate challenge models against Sudan ebolavirus (SUDV) and Marburg marburgvirus (MARV) infections.
Recent outbreaks have occurred in Africa, with increased frequency in 2023.
There are currently no approved vaccines or therapeutics for either SUDV or MARV infections.
However, vaccines are available for Zaire ebolavirus (EBOV) infections in 2024, but they provide no protection against SUDV or MARV infection.
"Filoviruses such as EBOV, SUDV, and MARV are some of the most lethal viruses known, and they are endemic in areas of the world where the power supply and distribution network can be uncertain, says the World Health Organization.
A thermostabilized vaccine in a single vial format would significantly enhance any public health response to a new outbreak, at its source," stated Axel Lehrer, Ph.D., Associate Professor, Department of Tropical Medicine, Medical Microbiology and Pharmacology, University of Hawaiʻi at Mānoa, in a press release.
"Our work to date has demonstrated the feasibility of rapid and efficient manufacturing, as well as the ability to thermostabilize multiple antigens that can then be stored for extended times at temperatures exceeding 100 degrees Fahrenheit."
"The use of a bivalent vaccine has the potential to both prevent future infections with these pathogens and potentially mitigate future outbreak events, potentially using an accelerated dosing regimen."
The thermostabilized filovirus vaccine program continues to advance with the support of a National Institute of Health grant and a Small Business Innovation Research grant awarded to Soligenix, Inc.

First identified in Germany in 1967, Marburg virus (MARV) outbreaks have been reported more than a dozen times over the past 56 years.
A Marburg virus (MARV) vaccine tested at Texas Biomedical Research Institute is progressing in clinical trials, moving a step closer to becoming the world’s first vaccine against the deadly virus.
The Sabin Vaccine Institute recently announced it launched Phase 2 clinical trials of its single-dose vaccine candidate ChAd3-MARV.
Early tests demonstrating the vaccine’s efficacy, safety, and optimal dosage were completed at Texas Biomed.
This announcement is essential since there are no approved Marburg vaccines or treatments.
“We have been partnering with Sabin since 2019 and are very excited to see their Marburg vaccine candidate move into Phase 2 clinical trials,” says Ricardo Carrion, Jr., Ph.D., the Director of Maximum Containment Contract Research at Texas Biomed, in a press release on December 7, 2023.
“An effective vaccine is critical to protect people from this deadly virus, especially as we see the frequency of outbreaks increasing in more places.”
The Phase 2 clinical trial will build on promising results from preclinical studies and a smaller Phase 1 clinical trial.
Texas Biomed continues to partner with Sabin to gather more detailed information that can only be gained through tightly controlled animal studies, including how soon protection is induced after vaccination.
Marburg virus is a part of the same filovirus family as Ebola virus and causes severe hemorrhagic fever. It is incredibly deadly, with up to a 90% fatality rate.
Recent MARV outbreaks that occurred in Equatorial Guinea killed 12 out of 17 confirmed cases, with another 23 probable deaths, according to the World Health Organization.
Tanzania also saw its first-ever Marburg outbreak, which killed six out of eight confirmed cases.
Texas Biomed has conducted similar work on Sabin’s closely related Sudan ebolavirus vaccine as part of a World Health Organization-coordinated outbreak response.
As of December 24, 2023, several Ebola vaccines, therapies, and vaccine candidates are conducting research studies. Ebola vaccines are not generally available in the U.S.
With approved Ebola vaccines and monoclonal antibody therapies available in Africa, a safe and effective treatment option is now the goal for researchers.
RedHill Biopharma Ltd. today announced that its two novel, oral host-directed investigational drugs, opaganib and RHB-107 (upamostat), demonstrated robust synergistic effect when combined individually with Veklury®, significantly improving viral inhibition while maintaining cell viability in a new U.S. Army-funded and conducted Ebola virus disease in vitro study.
Jeffrey Kugelman, Ph.D., Major(P), U.S. Army MSC, Branch Chief Synthetic Biology & Surveillance, Molecular Biology Division, U.S. Army Medical Research Institute of Infectious Diseases, who led the bioinformatics analysis of the study, commented in a press release on December 20, 2023, "The results suggest that opaganib and upamostat may have potential or use in combination with direct antiviral agents, such as Veklury, to improve treatment outcome, increasing efficacy while maintaining safety."
Opaganib (ABC294640) is a proprietary investigational host-directed and potentially broad-acting drug and is a first-in-class, orally administered sphingosine kinase-2 selective inhibitor with anticancer, anti-inflammatory, and antiviral activity, targeting multiple potential diseases.
"Opaganib is believed to be the first host-directed molecule to show activity in Ebola virus disease, and these results add to a recent U.S. Army Ebola virus study in which opaganib delivered a statistically significant increase in mice survival time in vivo," added Reza Fathi, Ph.D., RedHill's SVP R&D.
RHB-107 (upamostat) is a proprietary, first-in-class, once-daily orally administered investigational antiviral that targets human serine proteases in preparing the spike protein for viral entry into target cells. Because it is host-cell targeted, RHB-107 is expected to also be effective against emerging viral variants with mutations in the spike protein. In addition, RHB-107 inhibits several proteases targeting cancer and inflammatory gastrointestinal disease.
There are two types of Ebola causing outbreaks in Africa.
The initial Zaire Ebolavirus disease outbreak was confirmed in 1976 in South Sudan and the Democratic Republic of Congo.
African countries have endured Zaire Ebolavirus outbreaks between 2014, 2016, 2018, and 2022. Over 29,000 people were infected, and more than 11,000 died.
Separately, there have been five Sudan Ebolavirus outbreaks.
The World Health Organization issued new guidelines (August 2023) on Ebola infection prevention and control.

Since the discovery of the Ebola virus disease (EVD) in 1976, more than 30 outbreaks have been reported in Africa. While Ebola vaccines have been approved for adults, infants have not been protected from the EVD.
Published by The Lancet Global Health in November 2023, this analysis concluded an Ebola vaccine combination was well tolerated and induced strong humoral responses in infants younger than one year.
Furthermore, this phase 2 study concluded there were no safety concerns related to Zabdeno® (Ad26.ZEBOV) and Mvabea® (MVA-BN-Filo) vaccination.
And the reactogenicity profile comprised mild-to-moderate adverse events (grade 1 or 2). Within seven days of administration of either dose, there were no grade 3 solicited adverse events in the Ebola vaccine group.
The two-dose Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen induced robust antibody responses in 100% of infants 21 days after receiving dose two.
The serum antibody levels declined over the follow-up period, but 93% of the younger and 100% of the older infants were still considered responders 12 months post-dose 1.
In addition to its EMA-approved use in individuals aged one year or older, the results of the current Vaccines & Prevention B.V.-funded study could support the use of Ad26.ZEBOV and MVA-BN-Filo in infants aged 4–11 months, as recommended for off-label use in 2021.
As of November 30, 2023, access to Ebola vaccinations in the U.S. is limited. The U.S. CDC updated its Ebola Overbreak History on August 30, 2023.