Search API
Blue Water Biotech, Inc. today issued the following shareholder letter from its CEO, Dr. Neil Campbell.
As of October 30, 2023, Blue Water's vaccine development initiatives highlighted separate indications, such as chlamydia, influenza, malaria, and mpox.
The CEO's letter is excerpted below:
As we close out October, the first month of my tenure, I want to take this opportunity to personally communicate with you while providing an update on the direction of Blue Water Biotech.
As part of the shift in business strategy and to enhance shareholder value, the Company will focus on building a foundation of therapeutic, diagnostic, and service products in oncology that will bolster and enrich clinicians' medical practices.
.... the Company has established early-stage preclinical and clinical programs in various vaccine technologies. These vaccine programs were targeting a wide number of diseases and conditions that would have consumed an enormous amount of Company resources.
Considering the evolving market dynamics and post-pandemic challenges, we conducted a strategic and tactical assessment, which led us to conclude that the optimal path for the Company lies in de-prioritizing the vaccine programs to focus our efforts on expanding our oncology offerings.
In summary, our business strategy shift aims to enhance shareholder value by establishing a strong foothold in both therapeutic and diagnostic aspects of oncology.
The Company's SEC Report of unscheduled material events or corporate events on October 30, 2023, is linked here.

PharmaJet® recently announced the implementation of a study in Nigeria to evaluate the impact of intradermal vaccine administration of fractional inactivated poliovirus vaccine (fIPV) using their Tropis® ID Needle-free Injection System (NFIS).
Announced on October 24, 2023, the study, in collaboration with the National Primary Health Care Development Agency, Jhpiego, PATH, and the Sydani Group, is assessing coverage rates and potential cost reductions associated with using Tropis for fIPV delivery as compared to the current standard of intramuscular delivery of full dose IPV using needle and syringe.
Partners are also evaluating the acceptability and feasibility of using needle-free from the healthcare worker and caregiver perspective.
The study will run through January 2024, with children at 22 urban and rural health facilities receiving fIPV with Tropis. Evidence from the study is intended to inform policy regarding intradermal delivery of polio vaccine in routine immunization settings.
Paul LaBarre, Vice President of Global Business Development, PharmaJet, commented in a press release, “This (effort) builds on our experience in Pakistan where Tropis has been demonstrated to increase coverage rates. Including additional campaigns in Somalia, we have provided more than 10 million syringes for polio immunization programs."
"On this World Polio Day 2023, we restate our commitment to the Global Polio Eradication Initiative.”
As of October 30, 2023, the U.S. CDC confirms over thirty countries have reported polio outbreaks in the past year.
Araclon Biotech recently announced encouraging final results from its Phase 2 clinical trial of ABvac40, an active vaccine against the Aβ40 peptide, for treating patients with early-stage Alzheimer's disease (AD).
The results show that ABvac40 had a favorable safety profile, elicited a robust immune response against Aβ40, and demonstrated some potential cognitive benefits in early-stage AD patients.
The vaccine candidate met the primary endpoints and showed differences between the vaccine- and placebo-treated groups in some secondary exploratory endpoints.
ABvac40 is uniquely designed to target the C-terminal end of the Aβ40 peptide.
Thus, it is believed to prevent harmful reactions and avoid immune triggers responsible for meningoencephalitis, a complication observed in earlier AD vaccines.
Emerging research suggests that Αβ40 plays a role in cerebral amyloid angiopathy, a highly prevalent condition among the growing number of AD patients.
Notably, although the clinical trial was not powered for finding efficacy on neuropsychological scales, the ABvac40-treated group exhibited as much as a 38% reduction in disease progression, as reflected by the Mini-Mental State Examination score, suggesting ABvac40's potential efficacy in addressing the cognitive decline associated with AD.
Other neuropsychological tests, such as the Repeatable Battery for the Assessment of Neuropsychological Status and the Trial Making Test, showed favorable results on ABvac40 compared to the placebo group.
Global or functional scales did not show differences between the ABvac40 and placebo groups. In addition, volumetric magnetic resonance imaging showed a lesser increase in whole-brain atrophy in the ABvac40 group compared to the placebo group.
"We are pleased to report final positive results from the Phase 2 study of ABvac40, including a robust immune response with some significant reduction in disease progression, all with a favorable safety profile," said Jose Terencio, Ph.D., Araclon chief executive officer and vice president of Grifols Innovation and New Technologies, in a press release on October 25, 2023.
"Previous vaccines in development for AD faced setbacks due to harmful meningoencephalitis side effects."
"The results reported for ABvac40 to date validate its clinical potential, positioning it as a promising therapeutic candidate for early AD treatment. We look forward to evaluating the next steps for this program."
As of October 27, 2023, the U.S. FDA has not approved a vaccine targeting AD.

The National Institutes of Health recently awarded a $12.3 million contract to the University of Montana to develop a novel adjuvant for tuberculosis (TB) vaccine.
Adjuvants are integrated into numerous vaccines and are substances that boost their effectiveness.
The current leader in TB prevention is the 100-year-old Bacille Calmette-Guerin (BCG), whose various versions are used globally.
Merck's TICE® BCG vaccine is available in the U.S.
"The development and clinical evaluation of safe and effective adjuvants is urgently needed for the advancement of vaccines to combat the ongoing threat of bacterial and fungal infections, including tuberculosis, pertussis, and others," said Jay Evans, director of the UM center, in a press release on October 23, 2023.
Evans commented vaccine development for TB and other bacterial and fungal pathogens has been hampered by the lack of appropriate adjuvants and effective formulations.
"There is extraordinary research ongoing at UM that could positively impact the lives of countless people," Evans said. "Our Vaccine Research Team is dedicated to nurturing and cultivating an interactive research community at UM, specifically geared toward advancing these technologies to help individuals and communities in Montana and across the globe."
Drs. Evans and Walid Abdelwahab, along with their colleagues, are the co-principal investigators on the contract. The project includes researchers from the University of Chicago (Dr. Shabaana Khadar), the Texas Biomedical Research Institute (Dr. Smriti Mehra), and Missoula-based Inimmune Corp., a corporate development partner.
This new contract builds upon a recently completed $13 million NIH Adjuvant Discovery Contract, which identified the lead candidate being advanced toward human clinical trials in the current award.
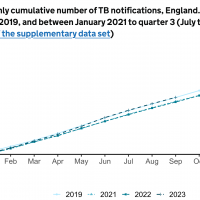
According to the World Health Organization (WHO), 1.6 million people died from TB in 2021. Worldwide, TB outbreaks are the 13th leading cause of death.
In 2020, almost two-thirds of TB cases in children worldwide were either not reported or went undiagnosed and untreated, according to the WHO.
Certain cities have recently reported increases in TB cases in the United States.
The U.S. CDC reported in March 2023 that TB cases increased by 5% in 2022, with 60 U.S. states, the District of Columbia, and territories provisionally reporting 8,300 TB cases.

The Federal Republic of Nigeria today announced the introduction of the human papillomavirus (HPV) vaccine into its routine immunization system.
The west African country of Nigeria aims to reach 7.7 million girls aged 9–14 in a vaccination drive against HPV types 16 and 18 that cause most cervical cancer cases.
As of October 24, 2023, this is the most significant number in a single round of HPV vaccination in the African region.
The second phase of the vaccination introduction is set to start in May 2024.
"The loss of about 8,000 Nigerian women yearly from a preventable (sexually transmitted) disease is completely unacceptable," says Muhammad Ali Pate, the Coordinating Minister of Health & Social Welfare, in a related press release.
A five-day mass vaccination campaign in schools and communities will be carried out during the inaugural rollout in 16 Nigerian states and the Federal Capital Territory.
The Federal Ministry of Health provides the vaccine for free through the National Primary Health Care Development Agency with support from Gavi, the Vaccine Alliance, United Nations Children's Fund (UNICEF), World Health Organization (WHO), and other partners.
WHO recommends that HPV vaccination be included in the national immunization programs of countries where cervical cancer is a public health priority and its cost-effective and sustainable implementation is feasible.
The Lancet Oncology reported in September 2023 that African countries are home to 19 of 20 countries with the highest burden of cervical cancer.
However, global supply shortages have slowed Gavi-supported HPV vaccine introductions.
These supply issues are now easing thanks to years of market-shaping efforts to develop a more robust HPV vaccine market and the single dose recommendation.
The Gavi board recently approved revitalizing its HPV vaccine program with an investment of over US$ 600 million by the end of 2025. With the additional funding, Gavi and its partners have set an ambitious goal to reach over 86 million girls by 2025, aiming to avert over 1.4 million future deaths from cervical cancer.
The WHO currently supports a one-dose regimen of the HPV vaccine for certain people.
The WHO's vaccine advisors recommend updating HPV dose schedules as follows: one or two-dose program for the primary target of girls aged 9-14, one or two-dose plan for young women aged 15-20, two doses with a 6-month interval for women older than 21, and immunocompromised individuals, including those with HIV, should receive three doses if feasible.
As of October 2023, there are effective HPV vaccines that protect men and women against cancers caused by HPV. These approved vaccines include 9vHPV, 4vHPV, and/or 2vHPV.

While two approved dengue vaccines are in use today, an innovative vaccine candidate was awarded $5.88 million to support activities to advance it into the clinic.
Codagenix Inc. announced on October 24, 2023, that the U.S. Department of Defense (DoD) awarded the Company $5,880,000 to advance the development of its CodaVax-DENV, a tetravalent live-attenuated dengue vaccine program.
The funds will support good manufacturing practices of drug substances and tetravalent drug product for a Phase 1 study, as well as a first-in-human Phase 1 safety and immunogenicity clinical trial.
This new award complements a $4.4 million DoD grant issued in 2022.
Codagenix's rational vaccine design platform is well-positioned to create a safe and effective dengue vaccine since its codon deoptimization process has the potential to provide immunity against all four dengue serotypes in a tetravalent formulation without the use of a backbone virus.
In addition to CodaVax-DENV, several dengue vaccine candidates are conducting clinical trials in 2023.
"We are honored to be selected for this award and would like to thank the DoD for their continued support, which is a recognition of the advantages of CodaVax-DENV, including codon deoptimization, homologous DENV 1, 2, 3, 4 strains, and the ability to titrate attenuation to develop balanced immunity," commented Jeffrey Fu, Ph.D., Chief Business Officer of Codagenix, in a related press release.
"These qualities uniquely position CodaVax-DENV to address the unmet needs in dengue prevention."
Dengue is a mosquito-borne viral infection, with over 2.4 billion people living in dengue-endemic areas worldwide; it is a leading cause of serious illness in several Latin American and Asian countries in 2023.
To alert international travelers of this health risk, the U.S. CDC recently issued Travel Health Notices regarding dengue outbreaks in the Americas (September 25, 2023), Africa/Middle East (October 18, 2023), Costa Rica, and Asia/Pacific Islands (July 25, 2023).
In the United States, Puerto Rico and Florida have reported the most dengue outbreaks in 2023.

The Janssen Pharmaceutical Companies today confirmed at the American Society of Tropical Medicine & Hygiene Annual Meeting positive results from a Phase 2a human challenge study evaluating JNJ-1802, a first-in-class oral antiviral in development for the prevention of dengue.
Announced on October 20, 2023, the data showed that the JNJ-1802 induced antiviral activity against dengue (DENV-3) in humans, compared to placebo, and is safe and well-tolerated.
JNJ-1802 is the first antiviral to show such activity in humans during a clinical trial.
The compound is not a vaccine and has advanced to a community-based field study to establish efficacy against circulating dengue serotypes in a real-world setting. The new research is being conducted in 30+ sites in 10 countries.
Marnix Van Loock, Ph.D., Lead for Emerging Pathogens, Global Public Health R&D at Janssen Pharmaceutica NV, stated in a press release, “Dengue requires global action, and we are proud to collaborate alongside partners around the world in advancing the development of this compound to its next phase.”
According to the U.S. CDC, dengue viruses are spread to people through the bite of an infected Aedes species. Annually, up to 400 million people get infected with dengue, and 40,000 die from severe dengue.
This new study follows data published in the journal Nature in March 2023, which showed that JNJ-1802 provides strong protection against dengue in non-human primates and mice.
And a Phase 1 first-in-human clinical study showed that the antiviral was safe and well-tolerated.
Globally, dengue outbreaks are active in numerous countries during 2023.
As of October 21, 2023, two dengue vaccines are approved for use in various countries.