Search API
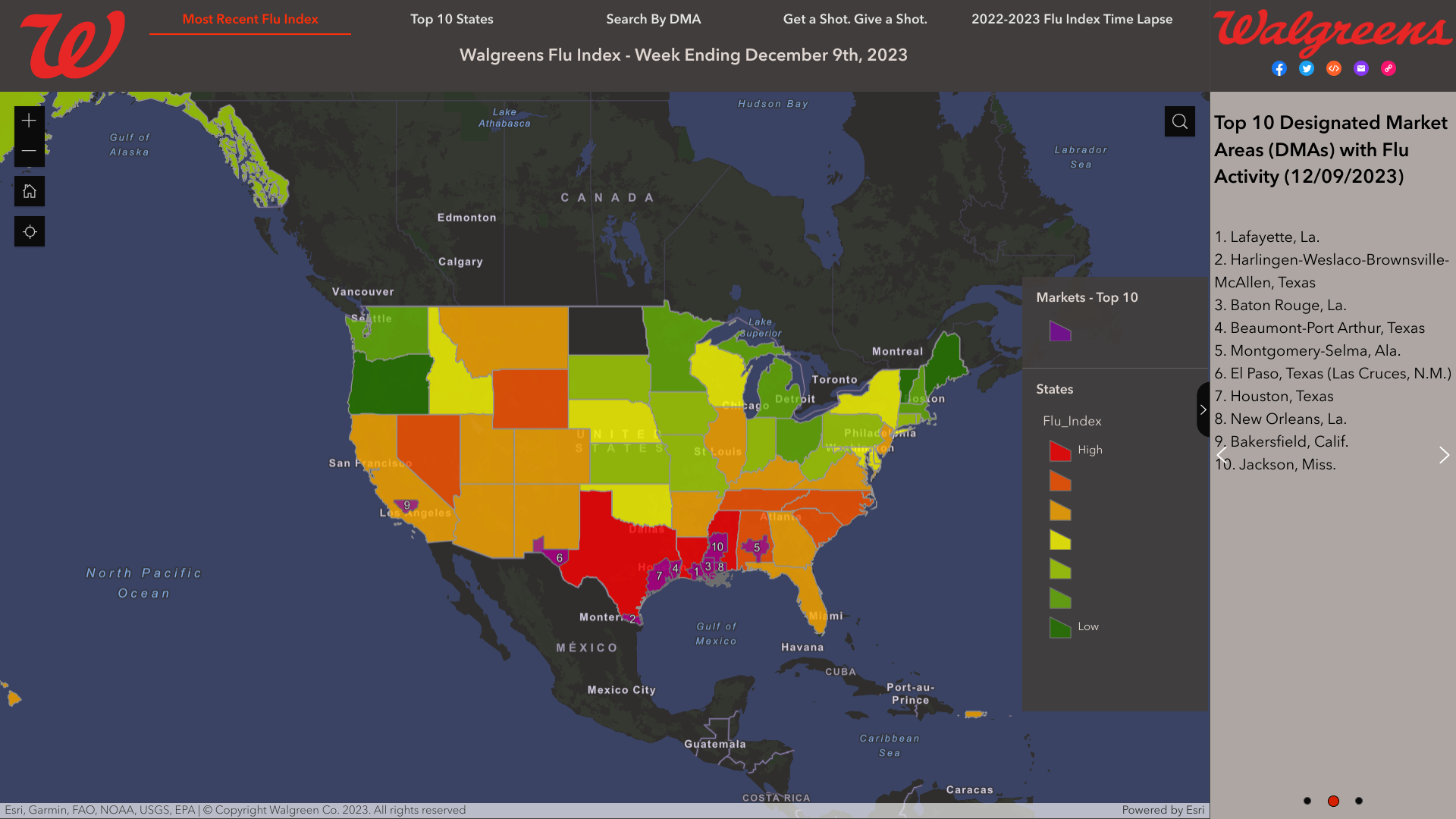
As national influenza rates increased post-Thanksgiving, according to the Walgreens Flu Index®'s latest report, the state of Texas is leading the nation in influenza activity.
As of December 9, 2023, the Index identified the leading cities using retail prescription data for antiviral medications used to treat influenza across Walgreens locations nationwide.
- Lafayette, La.
- Harlingen-Weslaco-Brownsville-McAllen, Texas
- Baton Rouge, La.
- Beaumont-Port Arthur, Texas
- Montgomery-Selma, Ala.
- El Paso, Texas (Las Cruces, N.M.)
- Houston, Texas
Anita Patel, PharmD, Vice President of Pharmacy Service Development at Walgreens, commented in a press release on December 7, 2023, ".... practicing good respiratory etiquette are all important steps to protect yourself and your loved ones this winter, especially if you are feeling sick or planning to travel and gather for the holidays."
Testing is the best way to know for sure if you have a specific respiratory virus so you can take appropriate precautions and get the proper relief or treatment immediately.
Once you know your test results, your pharmacist can help you determine the best next steps and get the appropriate treatment as soon as possible, whether that's a prescription medicine or over-the-counter essentials to manage your symptoms, says Walgreens.
Furthermore, the U.S. CDC recommends getting an annual flu shot before gathering with friends and family this holiday season.
As of December 2, 2023, over 152 million flu vaccines (nasal, cell-based, egg-based) have been distributed in the U.S. and are available at most clinics and pharmacies.
The Walgreens Flu Index is not intended to illustrate levels or severity of flu activity but rather to illustrate which populations are experiencing the highest incidence of influenza.
Icosavax, Inc. today announced it had entered into a definitive agreement under which AstraZeneca would purchase the company Phase 2 study of IVX-A12, a combination virus-like particle (VLP) vaccine candidate targeting both respiratory syncytial virus (RSV) and human metapneumovirus (hMPV).
There are currently no treatments or preventative therapies for hMPV. Adults with hMPV infection may have viral pneumonia, worsening asthma, or COPD symptoms. And there are no combination vaccines for RSV.
Announced on December 11, 2023, Iskra Reic, Executive Vice President, Vaccines & Immune Therapies, AstraZeneca, commented in a press release, "This VLP vaccine technology has the potential to transform prevention against severe infectious diseases, including RSV and hMPV."
"With the addition of Icosavax's Phase III-ready lead asset to our late-stage pipeline, we will have a differentiated, advanced investigational vaccine and a platform for further development of combination vaccines against respiratory viruses."
"This aligns with our strategy to deliver a portfolio of therapies to address high unmet needs in infectious diseases and our ambition to protect the most vulnerable patients who have a high risk of severe outcomes."
Separately, Icosavax announced positive topline interim results from its Phase 2 clinical trial of IVX-A12 against RSV and hMPV in older adults.
IVX-A12 induced robust immune responses against both RSV and hMPV at Day 28 across both formulations with and without adjuvant.
"We're delighted to announce positive topline interim data from our Phase 2 trial of IVX-A12, our potential first-in-class combination vaccine candidate against RSV and hMPV," said Adam Simpson, Chief Executive Officer of Icosavax, in a press release.
"We believe that IVX-A12 has the potential to address a significant unmet need and, as the furthest advanced RSV and hMPV combination vaccine in the clinic, to build on an emerging, large market opportunity."
The ongoing Phase 2 clinical trial of IVX-A12 is a randomized, observer-blinded, placebo-controlled, multicenter trial designed to evaluate the safety and immunogenicity of a single dose of RSV and hMPV combination VLP vaccine IVX-A12, with and without CSL Seqirus' proprietary adjuvant MF59®.
Regarding the proposed acquisition, the upfront cash portion of the consideration represents an equity value of approximately $838 million, a 43% premium over Icosavax's closing market price on December 11, 2023, and a 73% premium to Icosavax's volume-weighted average price for the preceding 60 trading days.
Combined, the upfront and maximum potential contingent value payments represent, if achieved, an equity value of approximately $1.1 billion, a 91% premium over Icosavax's closing market price on December 11, 2023, and a 130% premium to Icosavax's volume-weighted average price for the preceding 60 trading days.

Merck and Moderna, Inc. today announced the initiation of a pivotal Phase 3 randomized clinical trial (INTerpath-002) evaluating V940 (mRNA-4157), an investigational individualized neoantigen therapy, in combination with KEYTRUDA®, Merck’s anti-PD-1 therapy, as adjuvant treatment in patients with completely resected (R0) Stage II, IIIA or IIIB (with nodal involvement) non-small cell lung cancer.
Global recruitment of the INTerpath-002 has begun, and the first patients enrolled in Australia.
“As lung cancer is the leading cause of cancer death worldwide, there is a need for continued scientific advancements to help fight this disease at earlier stages when patients have the best chance for better outcomes,” said Dr. Marjorie Green, senior vice president and head of late-stage oncology, global clinical development, Merck Research Laboratories, in a press release on December 11, 2023.
“By combining KEYTRUDA with V940 (mRNA-4157), a promising new modality, we are researching innovative new approaches for earlier stage non-small cell lung cancer.”
As previously announced, in addition to INTerpath-002, the combination of V940 (mRNA-4157) plus KEYTRUDA is being investigated in INTerpath-001, a global, randomized, double-blind, placebo- and active-comparator-controlled Phase 3 trial evaluating patients with resected high-risk (Stage IIB-IV) melanoma.
INTerpath-001 is actively screening in 14 countries, representing 38 sites.
The companies confirmed they plan to expand the comprehensive clinical development program for V940 (mRNA-4157) to additional tumor types.

The Coalition for Epidemic Preparedness Innovations (CEPI) recently announced it had partnered with Jurata Thin Film, Inc. to advance development of thermostable under-the-tongue vaccine films as a needle-free vaccine delivery platform.
On December 5, 2023, CEPI confirmed that it will provide up to an initial $1.2 million to support Jurata's proprietary innovative formulation platform, which, if shown to be successful, could help expand access to vaccines in underserved regions and advance the global response to future emerging infectious disease outbreaks.
CEPI's initial funding will support optimizing the composition and process of creating thin films and preclinical studies.
Under the agreement with CEPI, Jurata will create vaccine films to remain stable at 2-8 degrees, 25 degrees, and 40 degrees.
Jurata will optimise the composition of the films by testing various buffers, pH, stabilizers, sugars, salts, and different drying parameters and assessing how this affects vaccine stability and delivery.
Jurata aims to improve vaccine accessibility by stabilizing the 3D structure of mRNA-containing lipid nanoparticle vaccine materials, provided by Quantoom Biosciences, part of Univercells, into a thin thermostable film, thereby removing frozen storage needs.
The vaccine films are also lightweight and compact, simplifying the transportation process and potentially allowing for more doses to be shipped at any one time compared to current needle-and-syringe distribution.
Dr. Irnela Bajrovic, Chief Scientific Officer, Jurata, commented in a press release, "Our stabilising formulations have the potential to facilitate global access to mRNA vaccines, and our thin film delivery platform could make vaccine administration far easier than needle-and-syringe injections."
"We are grateful to CEPI for supporting our innovative technology and look forward to working with Quantoom to show the breadth of mRNA vaccines our technology can stabilize and deliver."
This is the fourth partner to be announced as part of CEPI's Call for Proposals for thermostable vaccine manufacturing innovations, announced in January 2022.
Thermostable vaccines are also identified as a preferred vaccine characteristic by the World Health Organization.

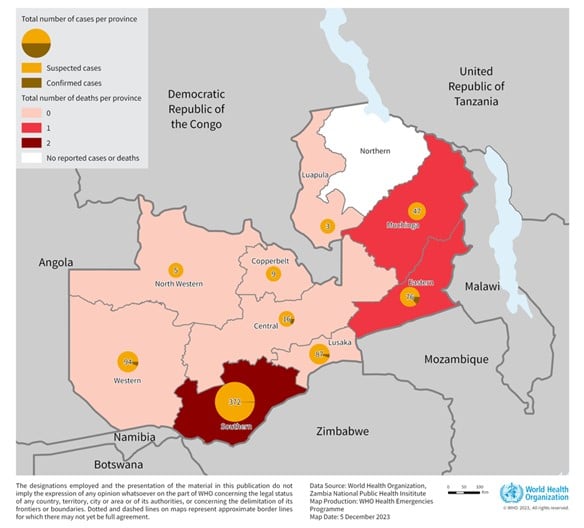
The ongoing anthrax outbreak in the Republic of Zambia has recently become a multi-country concern.
The World Health Organization (WHO today announced that as of November 20, 2023, 684 suspected human cases, including four deaths, have been reported in 2023.
This unprecedented anthrax outbreak marks the first significant occurrence spanning nine out of 10 country provinces. The last large-scale outbreak reported in Zambia occurred in 2011, with 511 suspected cases, wrote the WHO on December 8, 2023.
The risk of the outbreak spreading within Zambia is assessed to be high, and at the regional level is also considered high due to the frequent movement of both animals and people between Zambia and its neighboring countries, such as Angola, Botswana, the Democratic Republic of the Congo, Malawi, Mozambique, Namibia, Tanzania, Uganda, and Zimbabwe.
International travelers to anthrax-endemic countries should be aware of the current health risk, says the WHO. In 2021, about 554,000 tourists visited Zambia, formerly known as Northern Rhodesia, reported WorldData.
However, the WHO advises against implementing travel or trade restrictions with Zambia based on the current information on this event.
In addition to anthrax, the U.S. CDC has included Zambia in Travel Health Advisories in 2023 regarding measles and polio.
Humans usually acquire the infection after exposure to infected animals, carcasses, or animal products. More than 95% of human anthrax cases take the cutaneous form and result from handling infected carcasses or hides, hair, meat, or bones from such carcasses.
Anthrax is a zoonotic disease caused by Bacillus anthracis that typically affects ruminants (cows, sheep, and goats). The bacteria produce highly potent toxins responsible for the symptoms, causing a high lethality rate in the pulmonary form.
Humans can develop the disease from infected animals or through contaminated animal products. Hospitalization is required for all human cases identified. Vaccines are available for livestock.
However, humans have access to a limited supply.
From a prevention vaccination perspective, Emergent BioSolutions Inc. recently announced that the U.S. Biomedical Advanced Research and Development Authority awarded a $75 million contract option to acquire the newly licensed anthrax vaccine CYFENDUS™.
Deliveries of the two-dose vaccine are expected to begin in the U.S. in 2023 and be completed in the late first quarter of 2024. These vaccines are unavailable to the general public.
Anixa Biosciences, Inc. today announced new and updated positive results from the Phase 1 clinical trial of its breast cancer vaccine.
The data were presented by G. Thomas Budd, M.D., a staff physician at Cleveland Clinic Cancer Institute and principal investigator of the study, in a poster entitled "Phase I Trial of alpha-lactalbumin vaccine in high-risk operable triple negative breast cancer (TNBC) and patients at high genetic risk for TNBC."
Patients who had been curatively treated for TNBC received three vaccinations given once every two weeks. IFNγ and IL-17, which are T cell immune response indicators (cellular immunity), and antibody production (B cell humoral immunity) were measured to evaluate the vaccination effect.
Data from the 16 patients treated to date showed that:
Most patients developed ELISpot (T-cell) responses that met the rigorous protocol-specified definition of an immune response, with a measurable but lesser magnitude of response noted in the remaining patients.
12 (75%) of the women had antigen-specific IFNγ and/or IL-17 ELISpot responses at all dose levels, while ELISA antibody responses at Dose Level 2 and higher.
A statistically significant (P = 0.03) increase in IFNγ over baseline (Day 0) was observed by Day 56, while a significant (P = 0.0001) increase in IL-17 over baseline was observed by Day 14.
Among the doses studied, Dose Level 1 (10 mcg α-lactalbumin/10 mcg zymosan) was determined to be a usable immunologic dose and the maximum tolerated dose (MTD).
No significant side effects were observed at the MTD besides irritation at the injection sites. No myalgias, flu-like symptoms, or aberrant laboratory values were noted.
Anixa and Cleveland Clinic plan to investigate additional intermediate dose levels and continue studying the vaccine's safety and immunologic effects in two additional patient cohorts.
The first cohort, which opened for enrollment in August 2023, is evaluating the combination of the Company's breast cancer vaccine with Keytruda® (pembrolizumab) in post-operative patients found to have residual disease following neoadjuvant chemo-immunotherapy.
The second cohort will investigate the safety and immunologic effects of the vaccine in patients who are BRCA1, BRCA2, or PALB2 mutation-positive and are planning prophylactic risk-reducing mastectomies.
"The data from our Phase 1 trial has exceeded our expectations, and we are pleased with our progress. This vaccine is designed to direct the immune system to destroy TNBC cancer cells through a mechanism that has never previously been utilized for cancer vaccine development," stated Dr. Amit Kumar, Chairman and CEO of Anixa Biosciences, in a press release on December 7, 2023.
"We look forward to reviewing additional data as the trial continues to completion, and we are in the planning stages of the Phase 2/3 studies of this vaccine."
"Our goal is to initially evaluate the vaccine's ability to prevent recurrence of cancer in survivors and continue with extension studies to eventually determine its effectiveness in preventing the initial onset of TNBC."
Anixa is the exclusive worldwide licensee of the novel breast cancer vaccine technology invented at Cleveland Clinic, the site of the Phase 1 trial. The U.S. Department of Defense grant was made directly to the Cleveland Clinic.

As the United States heads into the winter months that typically coincide with peak respiratory illness season, Walgreens provides in-store and at-home testing and treatment options to help everyone stay healthy and feel better faster if they are experiencing symptoms.
"Respiratory illness activity and hospitalizations are picking up in many parts of the U.S., and these numbers are likely to continue increasing in the coming months," said Anita Patel, PharmD, Vice President of Pharmacy Service Development at Walgreens, in a press release on December 7, 2023.
"In addition to staying up to date on your vaccinations, getting tested, seeking treatment promptly, and practicing good respiratory etiquette are all important steps to protect yourself and your loved ones this winter, especially if you are feeling sick or planning to travel and gather for the holidays."
Walgreens stated testing is the best way to know if you have a specific respiratory virus so you can take appropriate precautions and get the proper relief or treatment immediately.
With influenza, RSV, and COVID-19 viruses circulating, visiting a local pharmacist in 2023 may be just what the doctor ordered.
Flu and RSV vaccines are covered by most insurance plans with a $0 copay and by Medicare and Medicaid in certain states.
Dr. Patel added, "People are increasingly relying on pharmacies as a one-stop destination for these services and deepening their relationships with their community pharmacists, who work tirelessly to provide the care and information they need all season long."
"As the nature of respiratory illness season continues to evolve post-pandemic, we remain focused on being a trusted partner in keeping our communities healthy."
As of December 2, 2023, the Walgreens Flu Index listed the top three states indicating influenza outbreaks:
- Louisiana
- Mississippi
- Texas
With nearly 9,000 retail locations across America, Puerto Rico, and the U.S. Virgin Islands, Walgreens is proud to be a neighborhood health destination serving almost 10 million customers daily.
Various types of flu shots are available as of December 7, 2023.

With the winter months ahead, most hikers are not focused on catching Lyme disease. However, once the snow melts, millions of people will once again not have access to a vaccine.
There are currently no approved human vaccines for Lyme disease.
To address this significant health risk, Pfizer Inc. and Valneva SE today announced that they have completed recruitment for the Phase 3 clinical trial Vaccine Against Lyme for Outdoor Recreationists (VALOR) for Lyme disease vaccine candidate VLA15.
The VALOR trial, initiated in August 2022, has enrolled 9,437 participants five years of age and older at sites where Lyme disease is highly endemic across the U.S., Europe, and Canada.
As part of the primary vaccination series, participants receive three doses of VLA15 or a saline placebo (1:1 ratio) within the first year and one booster dose approximately one year after completion of the primary immunization.
The trial builds on previous positive Phase 1 and 2 trial results and includes adult and pediatric participants to confirm the efficacy, safety, lot consistency, and immunogenicity of VLA15.
"Lyme disease is the most prevalent vector-borne infectious disease in the United States and Europe, can sometimes even lead to long-lasting consequences," said Annaliesa Anderson, Ph.D., Senior Vice President and Head Vaccine Research and Development, Pfizer, in a press release on December 4, 2023.
"If approved, a vaccine could prevent the disease and ease the burden of acute, severe, and sometimes persistent consequences in adults and children."
"We look forward to progressing the trial with the goal of submitting a Biologics License Application to the U.S. Food and Drug Administration and Marketing Authorization Application to the European Medicines Agency in 2026, subject to positive data."
VLA15 is an alum-adjuvanted formulation administered intramuscularly and has demonstrated a strong immune response and a satisfactory safety profile in pre-clinical and clinical trials.
This investigational multivalent protein subunit vaccine uses an established mechanism of action for a Lyme disease vaccine that targets the outer surface protein A (OspA) of Borrelia burgdorferi, the bacteria that cause Lyme disease.
OspA is a surface protein the bacteria expresses when present in a tick. Blocking OspA inhibits the bacterium's ability to leave the tick and infect humans.
The vaccine candidate covers the six most common OspA serotypes expressed by the Borrelia burgdorferi sensu lato species prevalent in North America and Europe.