Search API
Bavarian Nordic A/S, a leading pharmaceutical company, announced news today regarding its investigational chikungunya vaccine, CHIKV VLP.
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) has granted accelerated assessment for this chikungunya vaccine candidate's Marketing Authorisation Application (MAA).
The CHMP has recognized that the vaccine candidate is of significant interest to public health and therapeutic innovation.
With this positive development, the company is taking steps toward addressing the unmet medical needs of millions worldwide affected by the Chikungunya virus.
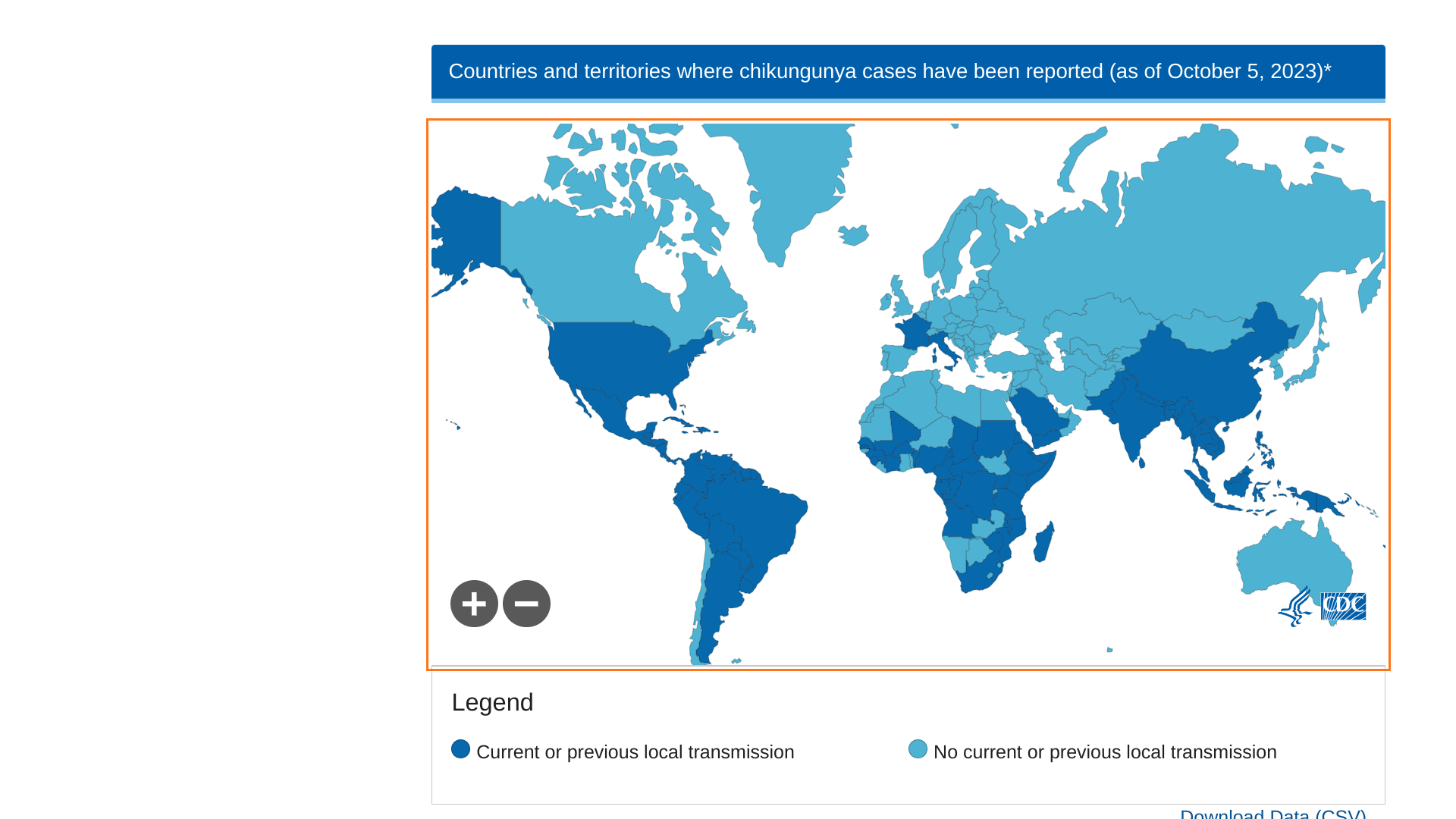
Chikungunya outbreaks continue to be reported in 2024.
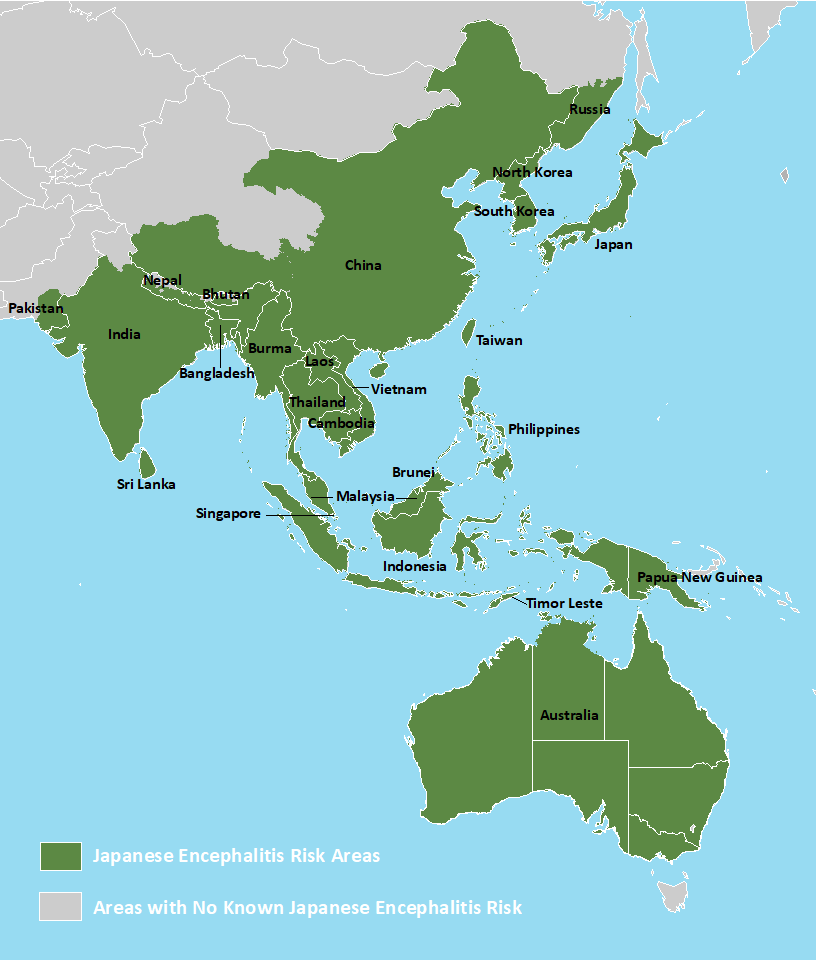
Before 2013, chikungunya virus cases and outbreaks had been identified in countries in Africa, Asia, Europe, and the Indian and Pacific Oceans. In late 2013, the first local transmission of chikungunya virus in the Americas was identified in Caribbean countries and territories, according to the U.S. CDC.
Bavarian Nordic also confirmed on February 23, 2024, that it is on track to submit its MAA for CHIKV VLP to the EMA during H1 2024. As a result, the review of the MAA may now take as little as 150 days instead of the usual 210 days.
This means that the vaccine could be available in Europe sooner than expected.
"We are pleased to receive the accelerated assessment in recognition of our chikungunya vaccine candidate and our efforts to bring this novel product to the market. With this, we can accelerate the approval and launch timelines for the vaccine in Europe. As part of our global strategy, we also plan to submit our biologics license application (BLA) for the vaccine candidate to the U.S. Food and Drug Administration later this year," said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release.
In 2023, Bavarian Nordic successfully completed two Phase 3 studies of CHIKV VLP.
The CHKV-VLA vaccine candidate received the U.S. Food and Drug Administration (FDA) Fast Track designation in May 2018.
Recently, the FDA issued approval for Valneva SE's IXCHIQ® Chikungunya Vaccine. However, the CDC has not given its approval.
Moderna Inc. today reported $2.8 billion in Spikevax® vaccine sales in the fourth quarter of 2023. The majority of Spikevax sales ($2 billion) were in international sales.
For all of 2023, Spikevax generated $6.7 billion in vaccine sales.
Moderna confirmed in a press release on February 24, 2024, that it achieved 48% cumulative market share in the U.S. retail segment during the fall 2023 COVID season, up from 37% in 2022.
The Company reaffirmed its 2024 product sales outlook as it entered the second year of the U.S. commercial endemic COVID market.
Moderna is also prioritizing key international markets for greater commercial focus and is participating in the EU Health Emergency and Response Authority's tendering procedure for up to 36 million doses of mRNA COVID-19 vaccines per year for up to four years.
As of February 2024, Spikevax is one of 13 COVID-19 vaccines Listed by the World Health Organization.
"2023 was a year of transition for Moderna as we adapted to the endemic market. At the same time, our development team made significant pipeline advancements across infectious diseases, oncology, and rare diseases, while our commercial team increased our COVID-19 market share in the U.S.," said Stéphane Bancel, Chief Executive Officer of Moderna, in a press release.

Brii Biosciences Limited today announced that it has entered into agreements with VBI Vaccines, Inc., ensuring expansion and control of future clinical and commercial supplies of BRII-179, a late-stage clinical asset in Brii Bio's HBV functional cure portfolio.
Brii Bio confirmed on February 13, 2024, that it will initially issue a $2.5 million promissory note to VBI. This will eliminate royalty and milestone payments for PreHevbri. The note will increase to $10 million upon meeting specific conditions, securing all of VBI's intellectual properties for BRII-179, with associated payments also eliminated.
In addition, subject to certain approvals, Brii Bio and VBI will work together to transfer the manufacturing technologies of BRII-179 to a site designated by Brii Bio.
Upon completing essential activities relating to such technology transfer, subject to certain potential adjustments, Brii Bio will issue up to an additional $8 million promissory note to VBI.
After satisfaction of certain conditions, Brii Bio will also take control of VBI's Rehovot-based manufacturing facilities for BRII-179 and PreHevbrio™ (PreHevbri®, Sci-B-Vac®) for $10 million cash on or after June 30, 2024, when Brii Bio and VBI plan to enter into supply agreement under which Brii Bio will become VBI's commercial supplier for PreHevbrio and PreHevbri.
Separately, subject to achievement of certain conditions by VBI, Brii Bio will secure an exclusive license to develop and commercialize VBI-1901, VBI's glioblastoma immunotherapeutic candidate, in the Asia Pacific region excluding Japan and issue a $5 million promissory note to VBI. VBI-1901 has received fast-track and orphan drug designations from the U.S. Food and Drug Administration and is conducting a Phase 2b study.
Dr. Zhi Hong, Ph.D., Chairman and Chief Executive Officer of Brii Bio, stated in a press release, "As Brii transitions to late-stage development of HBV programs, a global manufacturing strategy becomes critically important."
"We look forward to working with the biologics manufacturing experts at the Rehovot site and timely integration of our R&D and manufacturing capabilities."
According to the U.S. CDC, Hepatitis B is a vaccine-preventable liver infection caused by the HBV. It is spread when blood, semen, or other body fluids from a person infected with the virus enter the body of someone who is not infected. Not all people newly infected with HBV have symptoms, but for those that do, symptoms can include fatigue, poor appetite, stomach pain, nausea, and jaundice.

GSK plc announced today that the US Food and Drug Administration (FDA) has granted Fast Track designation for bepirovirsen, an investigational antisense oligonucleotide (ASO) for the treatment of chronic hepatitis B (CHB).
GSK said on February 12, 2024, Bepirovirsen is the only single agent in phase III development that has shown the potential to achieve clinically meaningful functional cure response when combined with oral nucleoside/nucleotide analogues (NAs).
The FDA designation was requested based on the potential for bepirovirsen to address an unmet medical need for CHB, a serious and life-threatening condition.
Data from the phase IIb trials B-Clear and B-Sure, which evaluated the efficacy, safety, and durability of the response of bepirovirsen in people with CHB, were submitted to support the application. A confirmatory phase III program, B-Well, is ongoing.
CHB affects nearly 300 million people worldwide, and current treatment options offer a less than 2-8% functional cure rate, which is not clinically meaningful.
Currently, available oral antiviral therapies only suppress the virus and do not directly lower hepatitis B surface antigen, which is essential for a functional cure.
Bepirovirsen is a triple-action investigational antisense oligonucleotide. It is also being investigated as a potential backbone therapy in future sequential regimens to pursue functional cures in a broader population of patients with CHB.
FDA Fast Track designation is intended to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need.
Bepirovirsen (previously known as 'ISIS 505358 or IONIS-HBVRX') was discovered and is jointly developed with Ionis Pharmaceuticals.
GSK is a global biopharma company that aims to unite science, technology, and talent to get ahead of disease together. Find out more at gsk.com. GSK's unedited press release is available at this link.

Valneva SE announced today that it recently sold the Priority Review Voucher (PRV) it received from the U.S. Food and Drug Administration (FDA) for $103 million.
The Company was awarded a tropical disease PRV in November 2023 following U.S. FDA approval of IXCHIQ®, Valneva's single-dose, live-attenuated vaccine indicated for preventing disease caused by chikungunya virus.
Under the Tropical Disease Priority Review Voucher Program, the FDA awards priority review vouchers to sponsors of tropical disease product applications that meet certain criteria. The program is intended to encourage the development of new drugs and biologics to prevent and treat tropical diseases.
PRVs can be redeemed to receive priority review of a subsequent marketing application for a different product, sold or transferred.
In a press release, Thomas Lingelbach, Chief Executive Officer of Valneva, commented, "This non-dilutive capital provides an important source of additional funding to advance the continued development of our clinical pipeline."
"As shown with the recent approval of our chikungunya vaccine, we remain committed to growing our portfolio of vaccines addressing unmet medical needs which have the potential to transform people's lives."
With the FDA's approval in 2023, IXCHIQ became the world's first licensed chikungunya vaccine to address this unmet medical need.

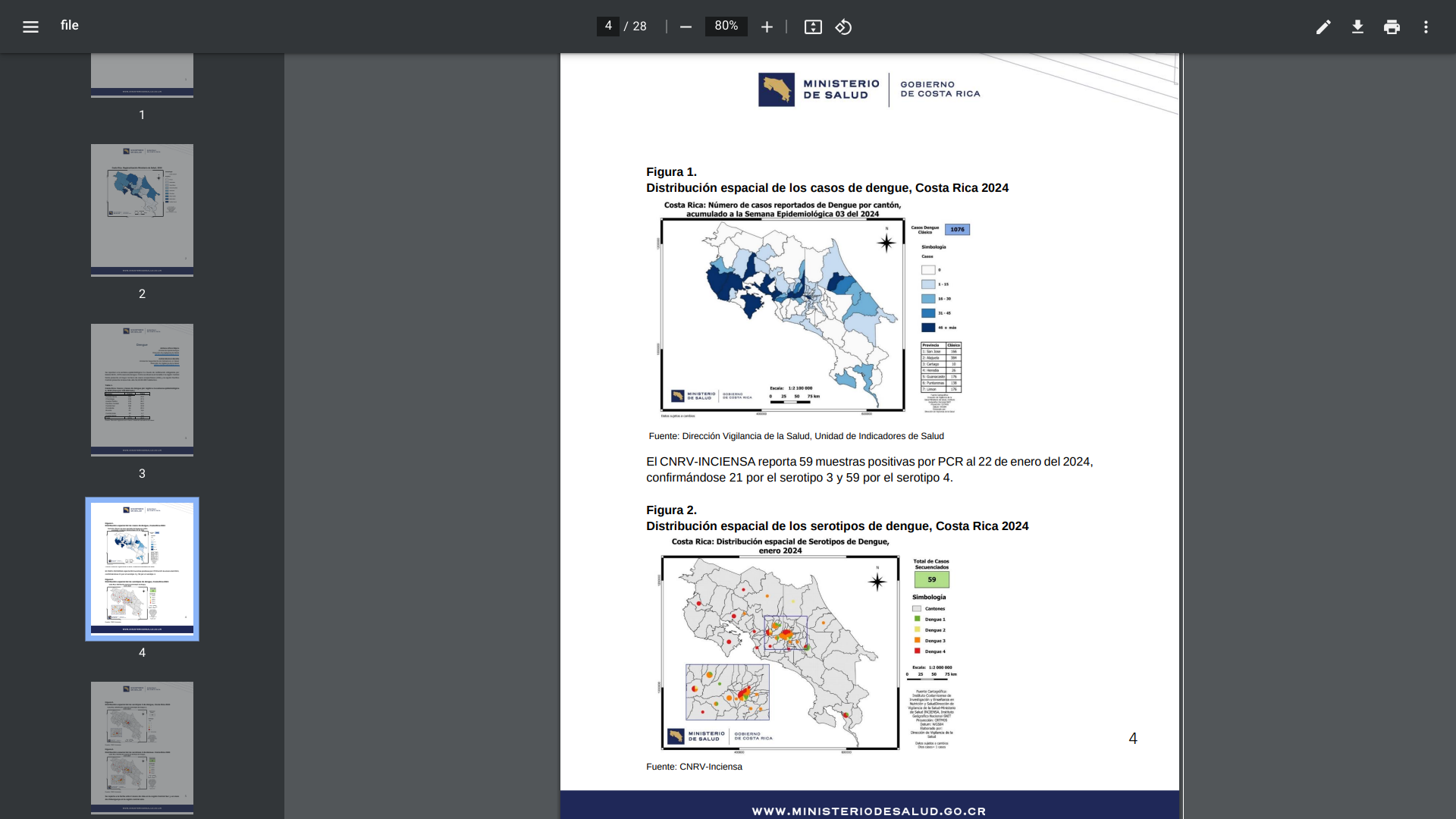
The Central American country of Costa Rica has been battling an outbreak of dengue fever, both classic and hemorrhagic, for the past few years. This mosquito-transmitted virus continues to impact Costa Rica residents and visitors in 2024.
On February 2, 2024, the Costa Rica Health Ministry reported that 1,076 dengue cases led by Central Norte have been confirmed this year.
Throughout 2023, there were about 24,914 dengue cases reported, a significant increase from the 7,485 patients in 2022.
Costa Rica is not alone in the Region of the Americas with the acceleration of dengue cases.
The Pan American Health Organization (PAHO) recently issued various dengue Risk Assessments that said dengue is endemic in most countries of South America, Central America, and the Caribbean. In 2023, the Americas experienced a 57% increase in dengue cases compared to 2022.
As of December 2023, the PAHO issued a Situation Report that assessed the risk of dengue outbreaks in the Americas as high at the regional level due to the widespread distribution of the Aedes spp. Mosquitoes.
In the U.S., the Centers for Disease Control and Prevention (CDC) has issued Travel Health Notices regarding dengue outbreaks in the Americas. The CDC reported on January 3, 2024, that there were 2,343 dengue cases reported by 52 U.S. jurisdictions during 2023.
The CDC says dengue is endemic in the U.S. territories of Puerto Rico, American Samoa, the U.S. Virgin Islands, the Federated States of Micronesia, the Republic of Marshall Islands, and the Republic of Palau.
Dengue is a vaccine-preventable disease, with two vaccines currently in use in the Americas and several dengue vaccine candidates in development in 2024.
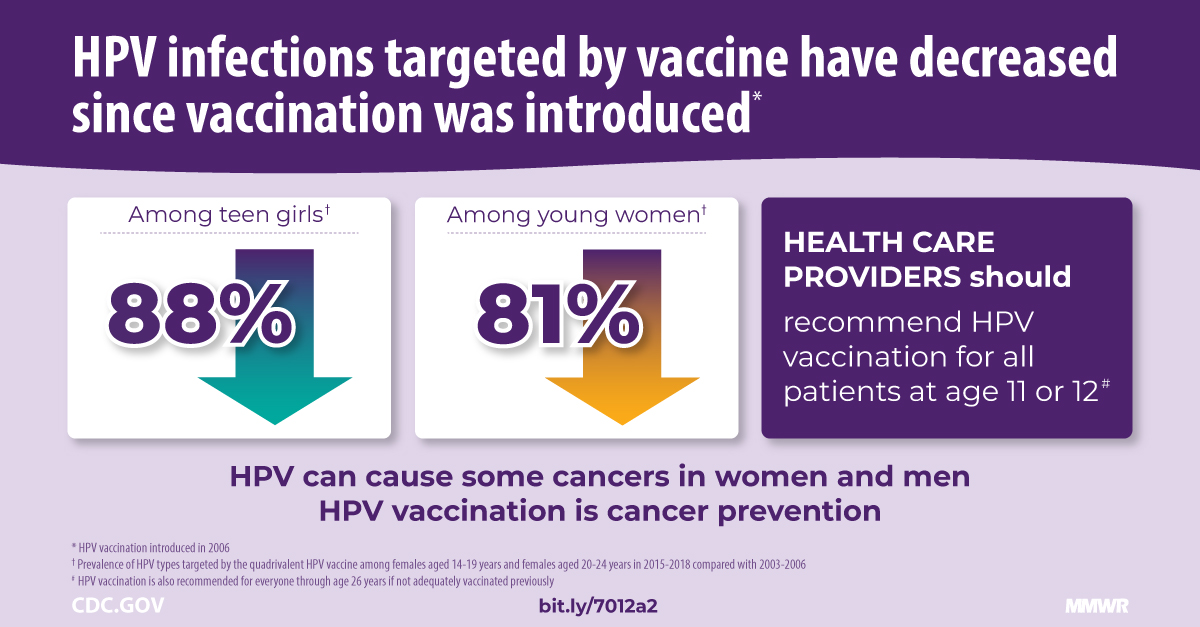
With Merck & Co. Inc.'s recent announcement, health leaders can expect a decrease in human papillomavirus (HPV) cancers in the coming years.
On February 1, 2024, Merck reported that GARDASIL / GARDASIL 9 HPV vaccine sales increased by at least 29% to reach $8.9 billion. This sales growth was due to strong global demand, particularly in China, and public-sector vaccine buying patterns in the U.S.
Robert M. Davis, chairman and chief executive officer of Merck, commented in a press release, "We also made investments of approximately $30 billion in research and development in our ongoing effort to discover, develop, and collaborate to propel the next generation of impactful innovations."
According to the U.S. CDC, getting an HPV vaccine for your child is the best way to protect them against certain types of cancer later in life.
Sexually transmitted cancers usually take years to develop after a person gets HPV. There is no way to know who will develop cancer or other health problems from HPV.
HPV can cause cancers of the cervix, vagina, and vulva in women, penis in men, and anus and throat in both men and women, says the CDC.
While Merck's GARDASIL 9® vaccine is a global market leader in 2024, approved vaccines are currently produced in India and China, with several next-generation vaccine candidates in development to address these vaccine-preventable cancers.
GlobalData confirmed today that Beyfortus™ (nirsevimab), a long-acting monoclonal antibody (mAb), has been approved in China for the prevention of respiratory syncytial virus (RSV) lower respiratory tract infection (LRTI) in neonates and infants entering or during their first RSV season.
With the first approved preventive option for RSV, AstraZeneca and Sanofi's Beyfortus will dominate the market in China, says GlobalData, a leading data and analytics company.
GlobalData's RSV Forecast in Asia-Pacific Markets (India, Urban China, Australia, South Korea, and Japan) to 2028 reveals that Urban China will lead the Asia-Pacific market for RSV in 2028, accounting for 34.8% of the overall market size.
Nelluri Geetha, Pharma Analyst at GlobalData, commented in a press release on January 31, 2024, "RSV infection is a leading cause of viral lower respiratory tract infections, with a higher rate seen in children than adults. RSV infection occurs most commonly in children below six months of age in China."
"Beyfortus is the first approved drug for RSV in a broad infant population, which includes healthy term, late preterm, and preterm infants, as well as infants with specific health conditions that make them vulnerable to severe RSV disease."
"Hence, the approval addresses an urgent need for novel prophylactic treatment options for the pediatric population in China."
Geetha concludes: "Beyfortus is the only preventive option for RSV in the infant population, meaning that the drug will continue to dominate the Chinese market shortly."
"However, competition may intensify over the long term as other drugs are in late-stage development for the pediatric population in this market. These include Merck & Co's clesrovimab and Zhuhai Trinomab Biotechnology's TNM-001 in Phase III development."
"These are mAbs in Phase III development for the prevention of RSV among pediatric patients."
As of February 1, 2024, Beyfortus is available in the U.S., U.K., and European markets for the 2024 RSV season. In 2023, Beyfortus sales reached €547 million in 2023.