Chikungunya Outbreaks
Chikungunya Outbreaks September 2025
Chikungunya virus (CHIKV) disease outbreaks have been recorded as early as 1824 in India. As of September 2025, the World Health Organization (WHO) reports that Chikungunya transmission has occurred frequently in approximately 119 countries, placing 5.6 billion people in Africa, Asia, the Indian Ocean, and the Americas at risk over the past decade. The WHO has published questions and answers about Chikungunya, including what it is, where it occurs, how to protect against it, its symptoms, treatment, and measures to reduce its spread.
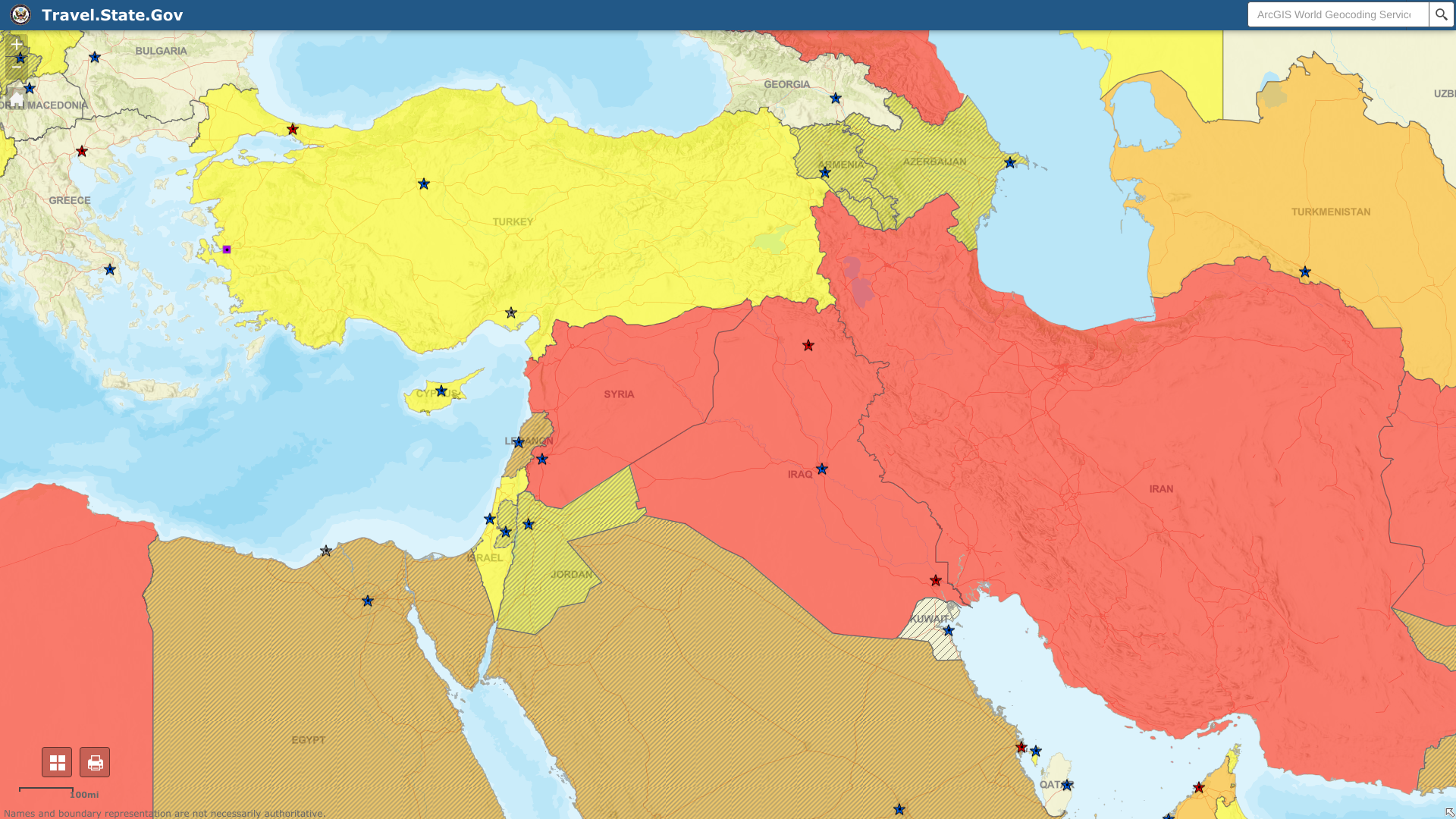
The U.S. Centers for Disease Control and Prevention (CDC) published Travel Health Advisories in 2025, which list countries and territories with evidence of CHIKV transmission among humans within the last five years. For example, in June 2025, the CDC issued a Level 2 Travel Health Advisory for the Plurinational State of Bolivia and the Indian Ocean region. France's Corsica, La Réunion, and Mayotte Departments, as well as Mauritius, Somalia, Sri Lanka, and the Maldives, are also experiencing significant CHIKV outbreaks in 2025. U.S. international travelers can use this information on chikungunya risk to help with vaccination decisions.
The European CDC reported that since the beginning of 2025, and as of July, approximately 90 CHIKV-related deaths have been reported in 16 countries/territories. Research published on June 10, 2025, estimates that 104 countries have experienced CHIKV transmission, affecting 2.8 billion people. In epidemic settings, the mean duration between outbreaks is 6.2 years, with 8.4% of the susceptible population infected with CHIKV per outbreak.
On August 5, 2025, a preprint study stated, 'Chikungunya epidemics are consistently associated with underrecognized mortality ( 1.34 (95% CI: 1.22–1.47; p < 0.000001) in April 2025.'.
Africa Chikungunya Outbreaks
Chikungunya cases are primarily located in Africa's Sahel region, where approximately 33 million people reside, including Senegal, The Gambia, Burkina Faso, Kenya, Mali, and Somalia. There have been ongoing or previous local transmissions in 2025. The Africa CDC reported over 1,900 cases of Chikungunya as of July 2025. As of February 2025, Senegal's Ministry of Health has reported Chikungunya cases in the Birkilane district of the Kafferine region. In August 2025, Nigeria issued a warning about the risk of a chikungunya outbreak.
A Review Article published in October 2024 disclosed Chikungunya cases in tropical Africa. The overall pooled prevalence of Chikungunya in East Africa was 20.6%. Subgroup analyses revealed that Rwanda and Djibouti exhibited high prevalence rates of 63% and 50.4%, respectively, while Kenya and Somalia reported a moderate prevalence of 12.2%. The Republic of Djibouti reported 8 CHIV cases among U.S. armed forces members between 2016 and 2022.
In 2023, the U.S. CDC published a Level 2—Practice Enhanced Precautions, Travel Health Advisory regarding chikungunya outbreaks in Burkina Faso. From 2019 to 2020, a large-scale Chikungunya outbreak occurred in the Republic of Djibouti.
Region of the Americas Chikungunya Outbreaks
As of August 19, 2025, the Pan American Health Organization (PAHO) reported over 212,029 Chikungunya cases and three related fatalities in the Americas this year, with the highest numbers in Argentina, Bolivia, Brazil, Paraguay, and Peru. In 2024, 431,408 CHIKV cases were reported by the PAHO.
As of August 2025, 50 cases of chikungunya had been reported in various states in the USA. As of December 2024 (Week 47), the U.S. CDC reported 173 Chikungunya cases in Territories and non-U.S. residents in 2024, led by Massachusetts (20), Texas (21), California, Colorado, Illinois, and New York. From 2006 to 2023, 4,590 travel-related CHIKV cases were reported in the U.S. in areas such as Florida and Puerto Rico.
Chikungunya was first reported in Argentina in 2016. In 2024, 425,138 CHIKV cases and 236 associated fatalities were reported in the Americas. Between 2013 and 2023, the PAHO reported more than 3.7 million CHIKV cases in the Americas. The PAHO reported 1,746 CHIKV cases in Argentina in 2023. A recent study traced the evolution of the virus in Argentina from 2016 to 2023.
The first case of Chikungunya in Bolivia was reported in early 2015. On August 15, 2025, the U.S. CDC reported an outbreak of Chikungunya in Bolivia and issued a Level 2 Travel Health Advisory. As of 2024, over 474 cases have been reported. In 2023, 1,455 cases were reported with no deaths, representing an 8-fold increase in patients compared to the same period in 2022. This study, concluded in October 2024, focused on seven years after the 2014-2015 CHIKV outbreak in Piedecuesta, Colombia, to determine the incidence of post-chikungunya chronic rheumatism (pCHIK-CR) and its impact on quality of life and chronic fatigue in adults. Chronic fatigue prevalence increased from 8.6% in patients without rheumatic Disease to 25% in non-inflammatory pain, likely degenerative, and 54.6% in pCHIK-CR cases.
Chikungunya outbreaks have been reported in Brazil since 2014. In 2025, Brazil's Ministry of Health published weekly arbovirus reports highlighting CHIKV cases, deaths, and locations. As of June 2025, the Ministry reported about 400,000 CHIKV cases. On March 18, 2025, the city of Xanxerê registered its second CHIKUNGUNYA-related fatality of the year. By the end of 2024, the PAHO reported over 420,139 CHIKUNGUNYA cases and 236 associated deaths in Brazil.
The highest-risk clusters were initially located in the northeast, dispersed to the central west and coastal areas of São Paulo and Rio de Janeiro (2018–2021), and then increased in the northeast (2019–2021). According to data from the Brazilian Vigilance Health Secretary, the three Brazilian states that have reported the most CHIKV cases are São Paulo, Pernambuco, and Paraíba. On October 28, 2024, a study reported that in 2023, during the Chikungunya epidemic in Minas Gerais, 890 excess deaths were attributed to the Disease, translating into a mortality rate of 35.1 per 100,000 inhabitants. The São Paulo dashboard was updated on February 25, 2025, indicating 2,063 confirmed cases and one CHIKV-related death in 2025. In March 2024, a study conducted at the São José do Rio Preto Medical School in São Paulo State, Brazil, revealed that the virus had been circulating in the city silently for years. An analysis of the blood samples showed that the number of Chikungunya cases in proportion to the population rose from 0.35% in 2015 to 2.3% in 2019. In February 2024, The Lancet Infectious Diseases published results from a study in Brazil that concluded the Chikungunya virus disease is associated with an increased risk of death for up to 84 days after symptom onset, including deaths from cerebrovascular diseases, ischaemic heart diseases, and diabetes. In 2021, São Paulo, Brazil's most populous state, saw a significant increase in cases, from 468 in 2020 to 18,156.
As of August 5, 2025, a total of eight confirmed locally acquired cases of chikungunya have been reported in Cuba in 2025. According to the Pedro Kourí Institute of Tropical Medicine, as of July 2025, a chikungunya outbreak has been reported in the Matanzas municipality of Perico.
Chikungunya cases have been reported in Equiador. CHIKV was introduced into Ecuador at multiple points in time between 2013 and 2014. These introductions were associated with the Caribbean. Our findings indicated no direct connection between CHIKV and Ecuador, Colombia, and Venezuela as of 2015.
In the eastern Caribbean, Grenada reported CHIKV cases in September 2024.
A study published in May 2025 suggests a 37% seroprevalence of Chikungunya virus in Paraguayan blood donors. In 2024, Paraguay reported over 2,700 cases. As of 2023, the PAHO reported 115,000 CHIKV cases in Paraguay. The U.S. CDC Advisory Committee on Immunization Practices workgroup presented "Update and Observations on a Large Chikungunya Outbreak in Paraguay" on June 22, 2023. From October 2022 to June 3, 2023, a total of 167,239 Chikungunya cases were reported. The CDC Health Alert Network issued CDCHAN-00487 on March 2, 2023, confirming that the Ministry of Health in Paraguay reported 71,478 suspected chikungunya cases in Paraguay since the outbreak began in October 2022. The East/Central/South African (ECSA) chikungunya genotype is circulating in the region. It was first identified in 2018 during an outbreak in the Amambay department and was subsequently detected in samples from 2022 in the Metropolitan Area of Asunción. As a result, an outbreak in Paraguay and surrounding countries is possible. In addition, the CDC reissued a Watch - Level 1, Practice Usual Precautions notice on April 6, 2023, confirming the presence of Chikungunya in the Asunción metropolitan area of Paraguay.
The first documented autochthonous transmission of the CHKV in the Caribbean island of Saint Martin was in 2013. Since 2014, Chikungunya outbreaks have been confirmed. As of March 2025, 3 CHIKV cases have been reported, and 16 cases were reported in 2024. Between 2014 and 2021, there were 221 confirmed Chikungunya cases and two deaths in Barbados
Asia Chikungunya Outbreaks
Chikungunya disease was initially reported in India in 1963. Since late 2005, the CHIKV has caused major outbreaks in Southeast Asian countries. In 2004, the CHIKV East/Central/South African (ECSA) genotype, characterized by E1: A226V and E1: K211E mutations, spread from Africa to the Indian Ocean islands and India, resulting in a large epidemic in Southeast Asia. A retrospective review of CHIKV cases in Southeast Asia found neurological manifestations or shock in 20% of hospitalized children. In India, CHIKV is one of the six critical vector-borne diseases in the National Vector Borne Disease Control Programme.
In 2025, the European CDC reported that India, Pakistan, Thailand, Timor-Leste, Myanmar, and Singapore had reported recent cases of Chikungunya disease. In 2024, the US CDC published an updated Level 2—Practice Enhanced Precautions —Travel Health Advisory regarding chikungunya outbreaks in various Indian states, including Maharashtra and Telangana. On January 2, 2025, India's National Center for Vector Borne Diseases Control reported 192,343 CHIKV cases in 2024 and 200,064 in 2023. As of 2024, Chikungunya has become endemic in every area of India. CHIKV cases have continued to be recorded in most states in India, such as Maharashtra (658) in May 2025. Pune's National Institute of Virology (NIV) is studying patient samples to determine if Chikungunya virus variants (Indian Ocean Lineage) contribute to the increase in cases and prolonged observed symptoms in 2024. Pune was among India's hotspot cities, recording 462 confirmed cases in 2024.
As of September 2025, the Institute of Epidemiology, Disease Control, and Research in Bangladesh has reported an outbreak of Chikungunya in Dhaka city, with 732 cases reported in 2025.
Karachi, Pakistan, experienced a Chikungunya outbreak in 2024. The Aga Khan University Hospital states that vaccination is the best way to prevent severe complications and fatalities associated with this virus in 2025. As of October 2024, 2.447 CHIKV cases have been reported in Pakistan.
The Republic of Singapore reported 19 CHIKV cases as of August 2025, 9 cases in 2024, and 13 cases in 2023.
Australia Chikungunya Outbreaks
Australia's National Notifiable Disease Surveillance System dashboard reported Chikungunya cases from 2019 to 2025. Queensland Health says autochthonous CHIKV cases do not routinely occur in Australia. Between 2002 and 2023, 26 cases of CHIKV were reported in Australia, with the infections acquired in Timor-Leste.
China Chikungunya Outbreaks
As of August 11, 2025, the Centre for Health Protection of the Department of Health reported that Foshan, a city in South China's Guangdong Province, confirmed a significant CHIKV outbreak in 2025. According to the information provided by Guangdong Province, the current CHIKV outbreak is active in the towns of Lecong, Beijiao, and Chencun in Shunde. The U.S. CDC issued a Level 2 Travel Health Advisory for Foshan, China, on August 1, 2025.
On August 10, 2025, the Centre for Health Protection of the Department of Health is investigating five imported cases of chikungunya fever (CHIKV) and a potential local case in Hong Kong. Between 2016 and 2019, the number of CF cases recorded in Hong Kong ranged from one to 11 each year. All of which were imported cases. There have been no CF cases in Hong Kong since 2020.
In August 2025, Macau's Health Bureau confirmed the city's first locally transmitted CF case and six travel-related cases this year.
In June 2025, the Taiwan CDC reported 19 CHIKV cases—16 of which were travel-related, compared to 20 cases in 2024.
Europe Chikungunya Outbreaks 2025
The geographic expansion of the Aedes aegypti (tiger) mosquitoes to more temperate regions in Europe in 2025 has increased the risk of arboviral disease outbreaks. As of August 2025, the European CDC assessment for CHIKV outbreaks was low. The ECDC states that the likelihood of local chikungunya virus transmission in the mainland EU/EEA is linked to the importation of the virus by viraemic travelers into receptive areas with established and active, competent mosquitoes. Past autochthonous outbreaks of CHIKV in mainland EU/EEA have occurred between June and November. Approximately 4,730 Chikungunya cases were documented across twenty-two countries in mainland Europe from 2007 to 2022.
As of August 12, 2025, Sante Publique France reported a total of 115 locally acquired and over 914 travel-related cases of Chikungunya in 2025. Cases have been reported from Provence-Alpes-Côte d'Azur, Corsica, Occitanie, Auvergne-Rhône-Alpes, Grand Est, and Nouvelle-Aquitaine. A press release from the Occitanie Regional Health Agency detected a locally transmitted Chikungunya case in the Hérault Department on June 16, 2025. An indigenous (locally) case was reported in La Crau (Var) on June 11, 2025, and in Île-de-France (Paris) in 2024. During 2024, 24 travel-related cases were reported in France. Two autochthonous cases were recorded in the commune of Cannet des Maures in the Var department in 2010, 11 cases in Montpellier in 2014, and 2 cases in the Var Department in 2017.
As of July 2, 2025, a third locally acquired case of Chikungunya has been detected in southern Corsica, in Porticcio (municipality of Grosseto-Prugna). In late June 2025, two instances of Chikungunya from the same family, residing in the city of Grossetto-Prugna, with no history of travel to tropical areas, were reported. ARS Corsica states that the presence of the virus-carrying tiger mosquito is now well established on the French island of Corsica.
As of August 2025, Italy's National Health Institute reported 66 confirmed cases of Chikungunya (37 travel-associated cases and 29 indigenous cases, but no deaths. Four local transmission events have been identified in Emilia-Romagna and Veneto. As of December 2024, Italy's National Institute of Health, Epidemiology for Public Health, reported 15 travel-related Chikungunya cases. A study published in December 2024 indicates that without vaccination, a CHIKV outbreak is estimated to infect up to 6.21% (170,762) of Rome's population. Travel-associated outbreaks led to CHIKV transmission in Italy in 2007.
An indigenous case of Chikungunya was confirmed in Hendaya, in the Basque Country, by the Basque Government's Department of Health in July 2025.
In 2024, 112 Chikungunya cases were reported in England (London: 43), Wales, and Northern Ireland (EWNI), more than double the 45 cases reported in the previous year. Most cases were linked to travel in Southern Asia, specifically India, where 66 cases were reported. This data represents about a 120% increase in EWNI compared to the same period in 2023.
Indian Ocean Chikungunya Outbreaks
According to the WHO and U.S. CDC, chikungunya outbreaks were active in Madagascar, the Maldives, Mayotte, Mauritius, Réunion, and Sri Lanka as of June 12, 2025.
Since August 2024, La Réunion Island has reported that Chikungunya has become endemic. As of May 12, 2025, about 47,000 confirmed cases and nine deaths have been reported in 2025. The municipalities of Étang-Salé and Le Tampon have the highest number of Chikungunya cases. In 2025, the U.S. CDC issued a Level 2 Travel Health Advisory regarding the Chikungunya outbreak in France's Réunion Department. France issued a Level 4 emergency for Réunion in March 2025. In 2024, Réunion reported 138 confirmed CHIKV cases, primarily in Étang-Salé, specifically in the Sheunon ravine district, with 70 cases. The last Chikungunya virus disease epidemic in La Réunion was in 2005–2006. Although CHIKV is generally transmitted by Ae. aegypti mosquitoes, the outbreak that occurred on La Réunion Island was caused by Ae. albopictus, which acted as the primary vector due to the ECSA CHIKV genotype's adaptation to this vector, specifically the E1-A226V mutation, resulting in a dramatic increase in infectivity.
During May 2025, six individuals who were infected with Chikungunya while visiting Madagascar were reported in France. Chikungunya outbreaks have been reported in Toamasina, Madagascar, since 2006.
Local authorities reported elevated Chikungunya activity in multiple areas of the Maldives, with over 300 cases reported in 2024. The U.S. CDC says there has been evidence of CHIKV transmission in the Maldives within the last five years. A significant Chikungunya outbreak occurred in 2019, with 1,736 cases reported. On May 28, 2024, the CDC issued a Level 2—Practice Enhanced Precautions, Travel Health Advisory for the Maldives.
Cases of Chikungunya, both imported and local, were detected in the Republic of Mauritius in 2025. As of March 17, 2025, Mauritius reported the first local case in the country since 2009. As of July 8, 2025, a total of 1,395 locally acquired chikungunya cases have been reported by the Africa CDC in Mauritius during 2025. According to the Mauritius health services, most chikungunya cases were imported from Asia, Africa, and the island of Réunion.
The French Department of Mayotte Regional Health Agency announced in June 2025 that 650 indigenous and travel-related cases had been confirmed in 2025. With the identification of the first indigenous Chikungunya case on Mayotte, health authorities activated level 2A of the ORSEC plan on March 26, 2025.
As of May 28, 2025, the UK Foreign, Commonwealth & Development Office (FCDO) issued travel advice about the risks of Chikungunya in Sri Lanka. As of March 2025, Sri Lanka reported 173 chikungunya cases in Colombo, Gampaha, and Kandy (22 cases in November and December 2024, and 151 cases during 2025).
Pacific Ocean Region Chikungunya Outbreaks
Chikungunya outbreaks have been reported in various countries in the Western Pacific Region. Large chikungunya outbreaks have been reported in the Philippines and Cambodia. Chikungunya outbreaks occurred in Malaysia in 1998-1999 and 2006. In 2024, over 72 cases were reported.
United Kingdom Chikungunya
UK Health Security Agency, dated August 14, 20indicatehows an increase in travel-associated chikungunya cases in England. A total of 73 cases were reported between January and June 2025. The same period in 2024 saw 27 cases. In 2025, the majority of cases reported travel to Sri Lanka, India, and Mauritius.
Chikungunya Vaccines
As of August 2025, the U.S. FDA and the EMA have authorized chikungunya vaccines, and clinical trials are accepting new participants.
Chikungunya Disease
According to the U.S. CDC, Chikungunya is a viral disease transmitted to humans through the bites of mosquitoes infected with the chikungunya virus (CHIKV). In 2013, the CHIKV Asian genotype drove an outbreak in the Americas, and Southeast Asian countries have detected the Chikungunya virus East/Central/South African-derived genotype with E1 mutations A226V and K211E. On January 20, 2025, the WHO published a Global Strategic Preparedness, Readiness, and Response Plan for Aedes-borne arboviruses and reported that Chikungunya mortality rates can vary from .01% to .05%.
The acute phase of the Disease caused by CHIKV begins shortly after the incubation period, which averages 2-7 days, and lasts up to fourteen days. In September 2024, an Original Article reported that Chikungunya-affected people experience damage to their physical and mental health, and positive screening for depression risk was 13.5 times more likely in chronically affected people. Patients with chronic chikungunya fever had a 76 times higher risk of walking impairments. In April 2024, the journal Cell Host & Microbe published results from a study, "Pathophysiology of Chikungunya Virus Infection Associated with Fatal Outcomes," which suggests that the Chikungunya virus crosses the blood-brain barrier, contributing to central nervous system infections.
Chikungunya encephalitis is a significant neurologic disorder of the central nervous system (CNS) with increased morbidity and mortality. As of 2025, a high index of clinical suspicion and aggressive management can lead to better outcomes.
The Lancet Infectious Diseases published results from a study in February 2024 that investigated the risk of death in people infected with Chikungunya two years after the first symptoms of the Disease appeared. Between 2015 and 2018 in Brazil, Chikungunya virus disease was associated with an increased risk of death for up to 84 days after symptom onset. The Lancet Infectious Diseases researchers published a study on February 8, 2024, that found the Chikungunya virus disease is associated with an increased risk of death for up to 84 days after symptom onset, including deaths from cerebrovascular diseases, ischaemic heart diseases, and diabetes. Data from 2015 to 2018 in Brazil revealed the incidence rate ratio (IRR) of death within seven days of chikungunya symptom onset was 8.40 (95% CI 4·83–20·09) as compared with the unexposed group and decreased to 2.26 in 57–84 days, and 1,05 at 85–168 days, with IRR close to 1 and wide CI in the subsequent periods.
Chikungunya Infection Impact on Neurodevelopment
A study published in January 2025 concluded that abnormal neurodevelopmental results were seen in both infected and uninfected children with intrauterine or perinatal CHIKV exposure.
Chikungunya Mortality Rate
In 2024, the ECDC reported about 620,000 CHIKV cases and 213 deaths were detected from countries in the Americas, Asia, Africa, and Europe, representing a 03% mortality rate. IN 2025, A case report disclosed discordant Chikungunya manifestations in a married couple, From acute undifferentiated fever to fatal sepsis with purpura fulminans.
Chikungunya-Carrying Mosquitoes
Over thousands of years, mosquito bites have caused more human suffering than any other organism. People can become infected with the chikungunya virus when mosquitoes feed on and bite an infected person. During the first few days of illness, people infected with the virus have high enough levels of the virus in their blood (viremia) to transmit it to mosquitoes. Recent studies published by the Royal Society and The Lancet indicate that disease-carrying mosquitoes are expanding their range by an average of 6.5 meters of elevation and have moved polewards by 4.7 km annually.