Search API
Vaxcyte, Inc. today announced that the U.S. Food and Drug Administration (FDA) granted Breakthrough Therapy designation for VAX-24, an investigational 24-valent pneumococcal conjugate vaccine (PCV) candidate for the prevention of invasive pneumococcal disease (IPD), in adults.
PD is an infection caused by Streptococcus pneumoniae bacteria.
With Breakthrough Therapy designation, Vaxcyte will have access to all of the elements of the FDA's Fast Track program.
The company stated the FDA's decision was based on positive topline results from the Phase 1/2 proof-of-concept study, which evaluated the safety, tolerability, and immunogenicity of VAX-24 in adults 18-64 years of age.
Grant Pickering, Chief Executive Officer and Co-Founder of Vaxcyte, said in a press release on January 5, 2022, "Our focus remains on advancing our VAX-24 clinical programs in both adults and infants."
"And we anticipate announcing the topline data from the Phase 2 study in adults 65 and older in the second quarter of 2023."
VAX-24 is intended to improve the standard-of-care PCVs for children and adults by covering the serotypes responsible for most of the pneumococcal disease currently in circulation.
Vaxcyte aims to efficiently create and deliver high-fidelity, broad-spectrum vaccines, such as VAX-24, by using modern synthetic techniques, including advanced chemistry and the XpressCF™ cell-free protein synthesis platform.
In the U.S., approximately 900,000 people get pneumococcal pneumonia each year, which is estimated to result in about 150,000 hospitalizations and 28,000 deaths.
Pneumococci also cause over 50% of all cases of bacterial meningitis, says the U.S. Centers for Disease Control and Prevention.
Antibiotics are used to treat pneumococcal disease, but some strains of the bacteria have developed resistance to treatments.
As of January 5, 2022, the U.S. FDA has approved various PCV vaccines, and several are conducting late-stage clinical research.
Disclosures: The company and CDC published the data, and this news post is not paid content.

Aura Air Inc. today announced the findings of an independent study confirming that the company's data-driven air purification devices are highly effective in eliminating the airborne pathogens that contribute to Respiratory Syncytial Virus (RSV).
A recent study by Innovative Bioanalysis Laboratory shows Aura Air's four-stage purification process filters and removes 99.997% of airborne RSV.
According to the U.S. Centers for Disease Control and Prevention, RSV results in approximately 58,000 annual hospitalizations.
Aura Air has partnered with schools, hospitals, and medical associations like the New Jersey Hospital Association (NJHA) to help them combat the spread of RSV, COVID-19, and the flu.
"Hospitals and communities across the country are being hit by an unprecedented surge in patients suffering from RSV, especially very young children," said New Jersey Hospital Association's SVP Michael A. Guerriero in a press release on January 4, 2023.
"Aura Air's advanced air purification and disinfection technology provide a welcome line of defense against these highly contagious viruses."
In addition to RSV, Sheba Medical Center, a leading Israeli medical facility, and Innovative Bioanalysis Laboratory confirmed that Aura Air successfully filters and removes 99.99% of airborne SARS-CoV-2 and 99.98% of Influenza A viruses.
Aura is headquartered in Israel with global offices in the U.S. and India, with distribution in about 87 countries.
Note: As of January 4, 2023, the U.S. FDA has not approved an RSV-preventive vaccine.
Disclosures: This content was sourced from the company and is not paid content.

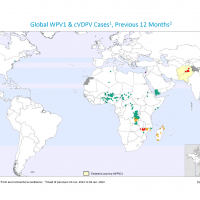
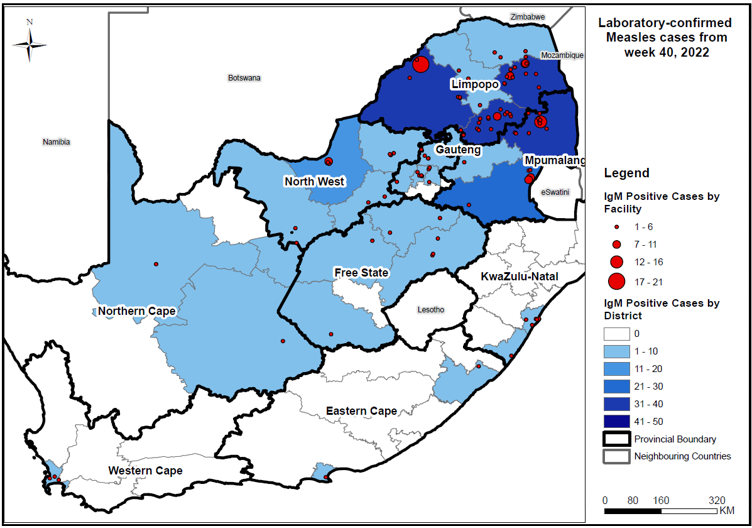
According to the latest National Institute for Communicable Diseases (NICD) report, South Africa's measles outbreak in children accelerated in late 2022.
As of December 29, 2022, 297 laboratory-confirmed measles cases have been reported in South Africa for specimens collected as of epidemiological week #51 across all provinces.
These measles cases were reported from five South African provinces: Mpumalanga (68 cases), North West (69 cases), Gauteng (13 cases), and Free State (7 cases).
The Western Cape, which includes the capital city of Cape Town, has only reported three cases.
South Africa's measles outbreak has been attributed to consistently lower than-optimal vaccine coverage of routine measles 1 and 2 doses.
"Ensuring that children are vaccinated against measles and other preventable childhood diseases is a matter of life or death," said Muriel Mafico, UNICEF South Africa Deputy Representative, in a press release on December 22, 2022.
"We call on all parents and caregivers to check the status of their children's immunization coverage and to get up to date as quickly as possible," added Mafico.
UNICEF also thanked the Governments of Germany and Japan for support of South Africa's vaccine cold chain, management, and risk communication.
As of January 4, 2022, the U.S. CDC has not issued a travel alert regarding South Africa's measles outbreak but does recommend MMR vaccinations for most visitors.
The CDC's Level 1 measles alert for Africa was issued on December 1, 2022, but did not include information about South Africa.
Various measles vaccines are available in the U.S. in most clinics and pharmacies.
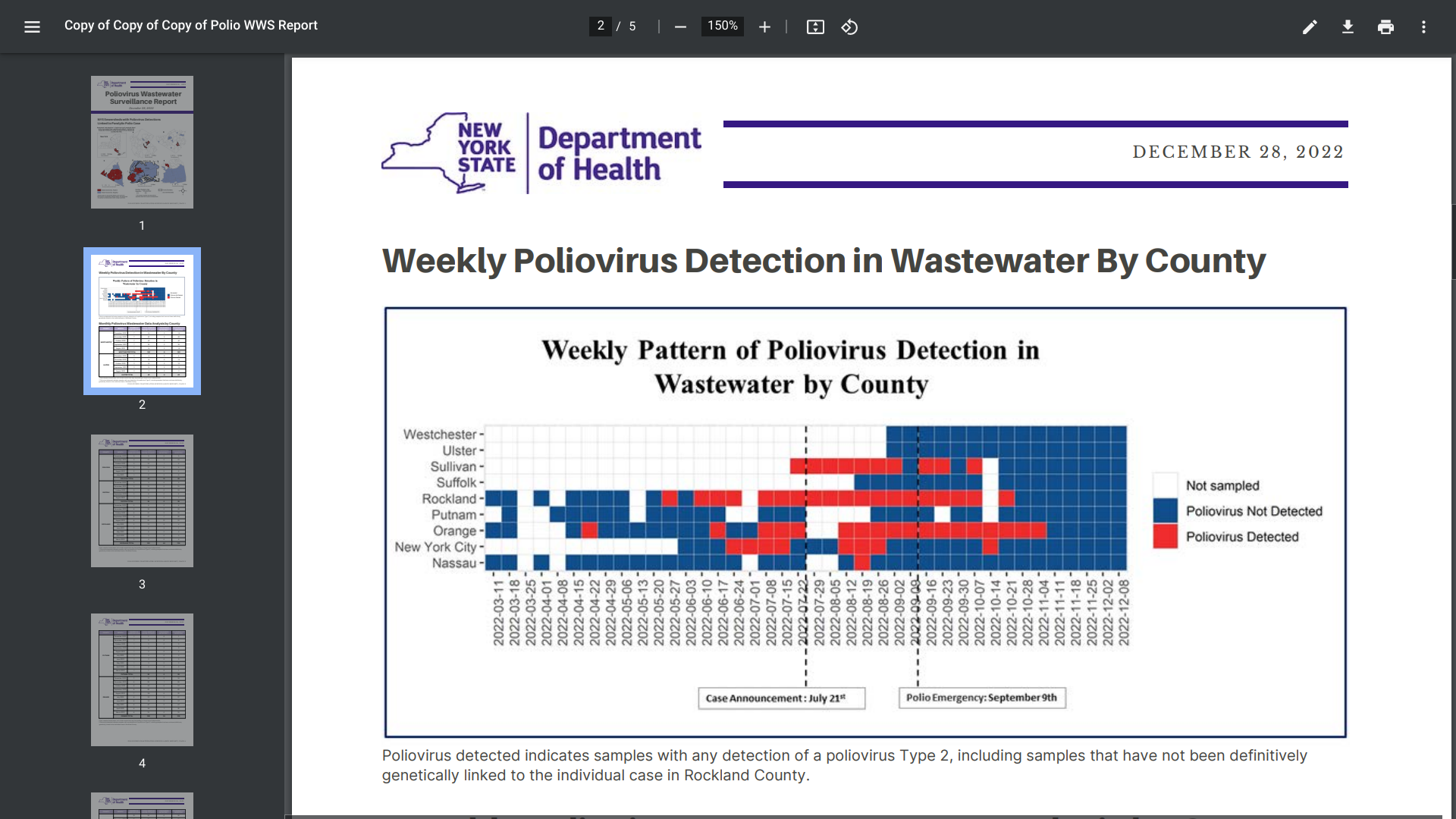
As 2022 ended, about thirty countries reported polio cases and/or poliovirus confirmations.
In response to these serious disclosures, the U.S. Centers for Disease Control and Prevention (CDC) reissued its Level 2 Travel Advisory.
On January 3, 2022, the CDC confirmed various international destinations have circulating poliovirus, such as London, England.
Since polio is a vaccine-preventable disease, the CDC says before any international travel, make sure you are up to date on your polio vaccines.
And the CDC recommends that adults who previously completed the full, routine polio vaccine series receive a single, lifetime booster dose of an authorized polio vaccine.
In the U.S., polio vaccines are generally available at most clinics and pharmacies.
While most people with polio do not feel sick, it can be a crippling and potentially fatal disease that affects the nervous system.
In reaction to a polio case confirmation in New York, the CDC announced on November 30, 2022, it would expand wastewater testing for poliovirus in select jurisdictions, such as near Detroit and Philadelphia.
As of December 30, 2022, sequencing analysis by the CDC confirmed the presence of poliovirus in a total of 99 positive samples of concern in New York.
Disclosures: Polio data originated from the CDC and NY Dept. of Health.