Search API
Situated in the Caribbean Sea, Grand Cayman is the westernmost of the three Cayman Islands. According to residents, three is a magic number.
When two is few and four is much, three islands are always just right, is a local saying.
And based on recent announcements, the U.S., Canadian, and U.K. governments agree with that saying.
For example, on January 10, 2023, the U.S. Department of State issued a low-level travel advisory for the Cayman Islands.
And if U.S. citizens need assistance, they must visit the U.S. Embassy in Kingston, Jamaica.
As the Cayman Islands is a British Overseas Territory, the U.K.'s Foreign Travel Advice recently confirmed there are no government requirements or limits on public gatherings in the Cayman Islands.
Since there's no formal British diplomatic or consular representation, local authorities deal with all requests for emergency assistance.
From a health perspective, the U.S. Centers for Disease Control and Prevention confirmed in November 2022 there are no health notices currently in effect for the Cayman Islands.
Furthermore, all vaccinated and unvaccinated visitors have been permitted to enter the Cayman Islands without additional documents since August 2022.
Travelers are not required to:
- Apply for a Travel Declaration
- Present a negative COVID-19 test
- Show proof of vaccination
- Wear masks
The Cayman Islands is conveniently about one hour flight south of Florida and is easily accessible via nonstop service from most travel hubs in the U.S., Canada, and the U.K.
George Town is the capital city, helping thousands of visitors enjoy their vacation. Besides seasonal hurricanes, the Cayman Islands is the place to relax in 2023.
Disclosures: This article is not paid content.

According to today's announcements, World Health Organization (WHO) and European Centre for Disease Prevention and Control (ECDC) officials again recommend airline travelers wear face masks while flying.
During a media briefing, Catherine Smallwood, WHO senior emergency officer for Europe, stated, "Passengers should be advised to wear masks in high-risk settings such as long-haul flights."
"This should be a recommendation issued to arriving passengers where there is widespread COVID-19 transmission."
The ECDC reported today the following recommendations apply with immediate effect for flights arriving in the EU from China:
- pre-departure testing for passengers on direct and indirect flights,
- wearing of medical face masks or respirators on board the aircraft for both passengers and crew,
- enhanced cleaning and disinfection of aircraft serving these routes,
- wherever possible, the vaccination status of crew members should be considered before assignment for duty,
- random testing may also be carried out on a sample of arriving passengers,
- wastewater should be monitored at airports with international flights and aircraft arriving from China to monitor the level of infection and detect any new variants.
These ECDC measures were defined in a way that should not introduce any flight delays or compromise flight safety.
While there is ample debate on the value of wearing face masks in the open air, numerous studies support mask-use when in closed settings, such as an airplane.
And the WHO Director-General's remarks at the media briefing on January 11, 2023, included, "And we continue to call on all people to take appropriate precautions when necessary to protect yourself and others."
"You may not die with this disease (COVID-19), but you could give it to someone else who does."
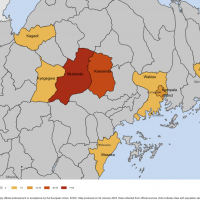
Globally, nearly 2.9 million new COVID-19 cases and over 11,000 related fatalities were reported for the week ending January 8, 2023.
However, the data represents a reduction in weekly cases and fatalities of 9% and 12%, respectively, reported the WHO today.

The U.S. Food and Drug Administration (FDA) has scheduled a Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting for January 26, 2023.
This digital meeting will start at 8:30 AM and is open to the public via YouTube https://youtu.be/ZjULNuSYfd0.
FDA confirmed it intends to make background material available to the public no later than two business days before the meeting. In addition, materials for this meeting will be available on the VRBPAC main webpage.
Furthermore, interested persons may present data, information, or views, orally or in writing, on issues pending before the Committee using this docket number: FDA-2022-N-2810.
Comments received on or before January 18, 2023, will be provided to the VRBPAC. Comments received after January 18, 2023, and by January 25, 2023, will be considered by the FDA.
Additional meeting information is available on this FDA webpage.
The VRBPAC reviews and evaluates data concerning the safety, effectiveness, and appropriate use of vaccines and related biological products intended to prevent, treat, or diagnose human diseases.
And, as required, any other products for which the FDA has regulatory responsibility.
The Committee also considers the quality and relevance of the FDA's research program, which provides scientific support for regulating these products and makes appropriate recommendations to the Commissioner of the FDA.

The World Health Organization (WHO today issued a Rapid Risk assessment regarding the XBB.1.5 coronavirus variant. The XBB.1.5 variant is a sublineage of XBB, which is a recombinant of two BA.2 sublineages.
Based on its genetic characteristics and early growth rate estimates, XBB.1.5 may contribute to increases in case incidence.
As of January 11, 2023, the WHO stated in a media release that the overall confidence in the assessment is low, as growth advantage estimates are only from one country, the United States of America (USA).
From October 22, 2022, to January 11, 2023, 5,288 sequences of the Omicron XBB.1.5 variant have been reported from 38 countries.
Most of these sequences are from the USA (82.2%), the United Kingdom (8.1%), and Denmark (2.2%).
Globally, nearly 2.9 million new COVID-19 cases and over 11,000 related fatalities were reported for the week ending January 8, 2023.
This Edition 125 data represents a reduction in weekly cases and deaths of 9% and 12%, respectively, reported the WHO on January 11, 2023.
The U.S. Federal Aviation Administration (FAA) today announced at 0850 EST that normal air traffic operations are resuming gradually across the United States following an overnight outage to the FAA’s Notice to Air Missions (NOTAM) system.
The NOTAM provides safety information to flight crews when flying in the U.S.
Before commencing a flight, pilots are required to consult NOTAMs, which list potential adverse impacts on flights, from runway construction to the potential for icing.
Furthermore, the FAA is in the process of modernizing the NOTAM system to improve the delivery of safety-critical information to aviation stakeholders.
Earlier on January 11, 2023, the FAA confirmed departures were resuming at Newark Liberty and Atlanta Hartsfield-Jackson airports due to air traffic congestion in those areas.
The AP reported about 21,000 flights were scheduled for departure today.

Regeneron Pharmaceuticals, Inc. discretely revealed its intentions to expand its Anti-SARS-CoV-2 Monoclonal Antibody offerings at the J.P. Morgan Healthcare Conference on January 9, 2023.
Regeneron's presentation stated that in the U.S. alone, millions of immuno-compromised people will not adequately respond to vaccination.
And antibodies can be dosed prophylactically to prevent infection and severe COVID-19 disease.
On slide #27, the Company stated its 'Next-gen COVID antibody binds outside variable RBD and has demonstrated high neutralization activity against all known variants and lineages and disclosed it anticipates initiating the REGN14287 phase 3 clinical trial in 2023, pending regulatory discussions.
The NCT04425629 study was last updated on July 29, 2022.
Previously Regeneron's first-generation REGEN-COV monoclonal antibody combination (casirivimab and imdevimab) is U.S. Food and Drug Administration-approved and was a market leader in the U.S.
The U.S. NIH stated on December 1, 2022, vaccination remains the most effective way to prevent SARS-CoV-2 infection and should be considered the first line of prevention.