Search API
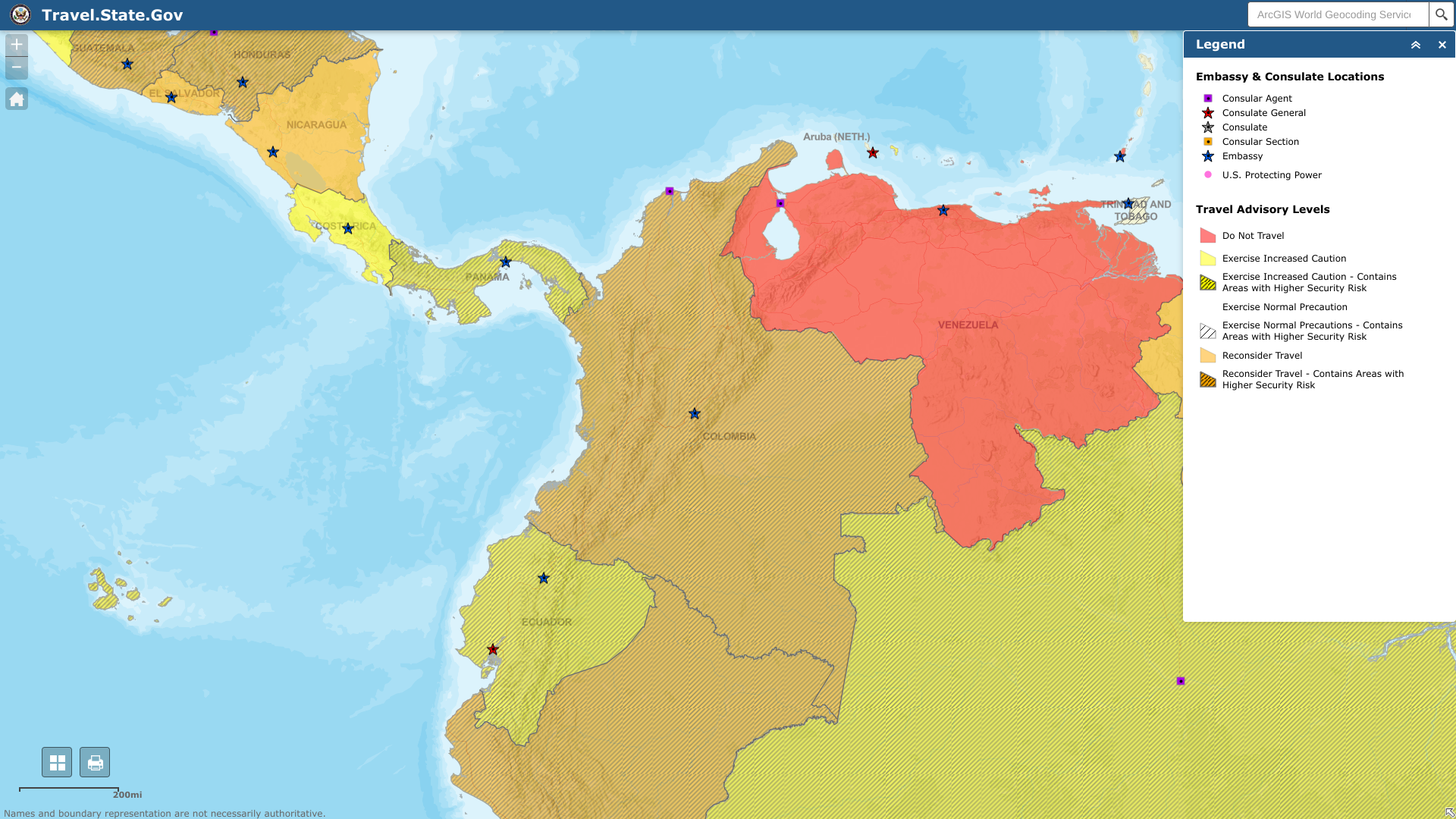
The U.S. Embassy in Bogota recently published a Level 3: Reconsider Travel notice with updates to high-risk areas. As of January 5, 2023, Columbia's U.S. Embassy says to exercise increased caution due to civil unrest.
Specifically, U.S. citizens are advised not to visit Arauca, Cauca (excluding Popayán), and Norte de Santander departments.
And the Colombia-Venezuela border region.
Furthermore, demonstrations occur regularly throughout the country. As a result, road closures may significantly reduce access to public transportation and disrupt travel within and between cities.
As a result, U.S. government employees are not permitted to travel by road between most major cities.
And Colombia's land border areas are off-limits to U.S. government personnel unless authorized.
If you decide to travel to Colombia, the State Department suggests keeping a low profile and enrolling in the Smart Traveler program to receive digital alerts and make it easier to be located during an emergency.
And U.S. citizens can obtain local assistance at U.S. Embassy in Bogota, at Calle 24 Bis No. 48-50, Bogotá, D.C. Colombia.
From a health perspective, the U.S. Centers for Disease Control and Prevention (CDC) included Columbia in its Dengue outbreak travel advisory.
Furthermore, the CDC suggests various travel vaccinations, such as malaria, measles, and yellow fever, before visiting Columbia.
Travel vaccines are available in the U.S. at certified clinics and pharmacies.
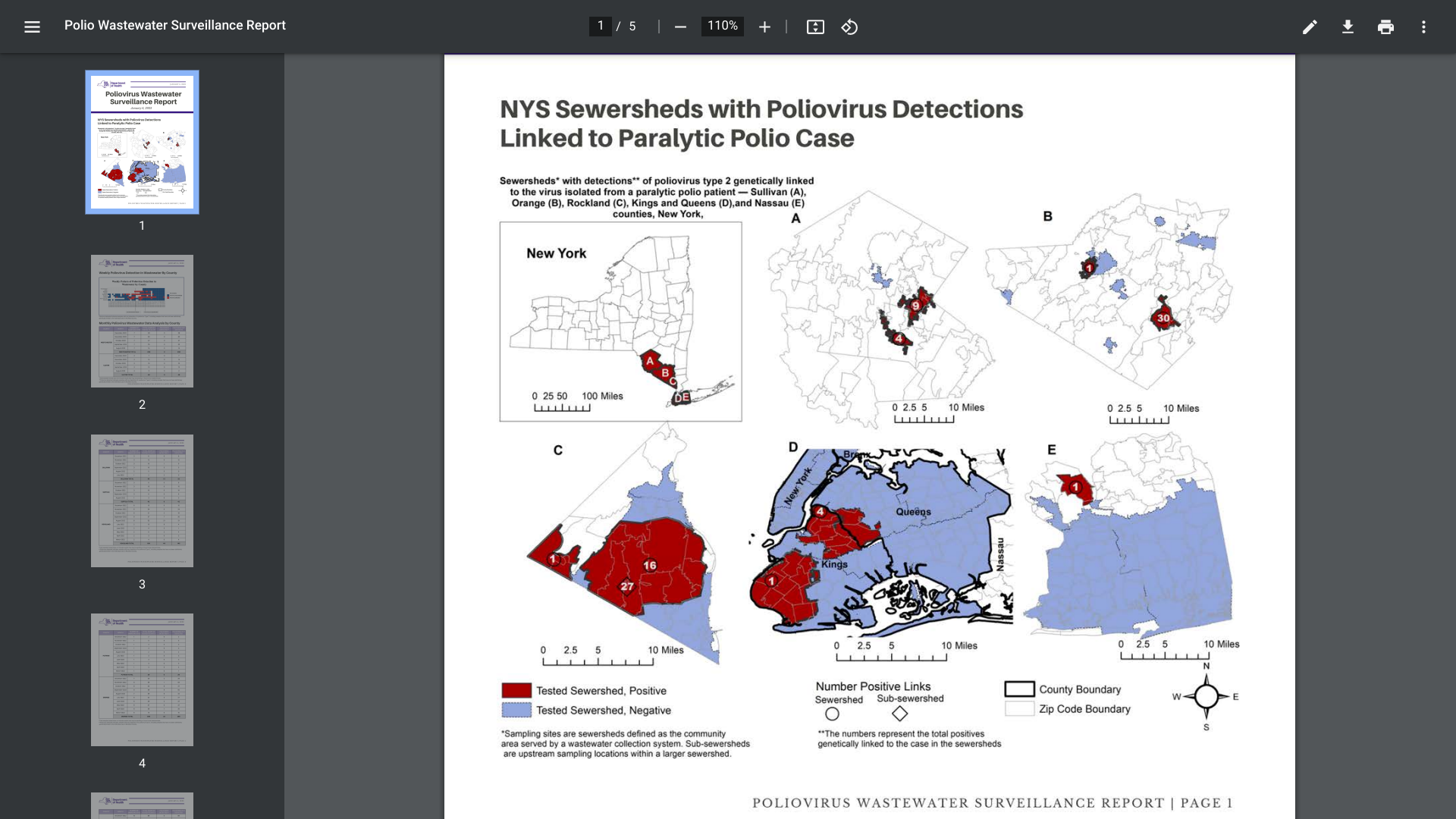
The New York State Department of Health Wastewater Surveillance's recent report reveals two additional positive poliovirus samples were identified in lower New York.
As of January 6, 2023, sequencing analysis by the Centers for Disease Control and Prevention (CDC) confirmed the presence of poliovirus in a total of 101 positive samples of concern.
The poliovirus samples were collected in Rockland County, Orange County, Sullivan County, Nassau County, Brooklyn (Kings County), and Queens County.
The CDC says 94 of these samples are genetically linked to a Rockland County, NY, resident who was confirmed with polio (Acute flaccid myelitis) in 2022.
Polio is highly contagious, and people can spread the virus even if they don't know they're sick. However, individuals infected with polio shed viruses in their stools.
New York says wastewater surveillance is an essential public health tool, providing the early and ongoing detection of polio in communities.
This monitoring helps identify where the virus may be and when, though it does not provide quantitative information about who or how many people or households may be infected.
Furthermore, wastewater collected in sewer systems in New York cannot be a source of polio infection or transmission for the general public. And it does not contaminate New York drinking water, including tap water, streams, and lakes, says the Department of Health.
The CDC says polio is a vaccine-preventable disease, and vaccines are generally available at clinics and pharmacies in New York.
Other polio outbreak news for 2023 is posted at PrecisionVacccinations.com/Polio.
When BioNTech SE announced that the first person was dosed in a first-in-human Phase 1 clinical trial with BNT163, a herpes simplex virus (HSV) vaccine candidate for the prevention of genital lesions caused by HSV-2 and potentially HSV-1, there was much enthusiasm generated by millions of people.
BioNTech's placebo-controlled, observer-blinded, two-dose-escalation study was launched on December 8, 2022, and is expected to enroll around 108 healthy adult volunteers.
This first-in-human HSV vaccine study was last updated on January 5, 2023.
However, it is scheduled for a June 2025 completion date.
BNT163 is not the only herpes vaccine candidate conducting early-stage research.
Still, it is the only mRNA vaccine that encodes three HSV-2 glycoproteins to help to prevent HSV cellular entry and spread, as well as counteract the immunosuppressive properties of HSVs.
"My colleagues and I are proud to have contributed to the early development and preclinical testing of this exciting new mRNA vaccine candidate that may have the potential to prevent people from contracting the virus," commented Prof. Harvey M. Friedman, M.D., Professor of Infectious Diseases at the University of Pennsylvania's Perelman School of Medicine, in a press release.
Dr. Friedman conducted preclinical and discovery science work on HSV and is the University's principal investigator for the preclinical discovery and enabling studies.
The U.S. Centers for Disease Control and Prevention (CDC) recently estimated that there were 572,000 new genital herpes infections in a year.
Throughout the U.S., about 12% of persons aged 14 to 49 have an HSV-2 infection.
From a herpes treatment perspective, research recently indicated about 15 vendors were participating in the herpes treatment market, which is valued at over three billion dollars annually.
Acyclovir and Valtrex® have significant shares in the pharmacy segment.
And United BioPharma is developing UB-621 as a first-in-class anti-gD monoclonal antibody candidate with demonstrated viral suppression of transmission and recurrence of HSV-1 and HSV-2.
This HSV antibody candidate's phase 2 study was last updated on May 18, 2022.
Updated herpes vaccine candidates and treatment news are posted at PrecisionVaccinations.com/Herpes.

The U.S. Food and Drug Administration (FDA) recently approved Eisai R&D Management Co., Ltd.'s Leqembi for treating Alzheimer's disease.
Leqembi (lecanemab-irmb) is the second of a new category of medications approved for Alzheimer's disease that target the fundamental pathophysiology of the disease.
In July 2021, the FDA approved Aduhelm, an amyloid beta-directed antibody indicated to treat Alzheimer’s disease.
These medications represent an essential advancement in the ongoing fight to effectively treat Alzheimer's disease.
The labeling states that treatment with Leqembi should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was studied in clinical trials.
The labeling also states that there are no safety or effectiveness data on initiating treatment at earlier or later stages of the disease than were studied.
The FDA granted this application Fast Track, Priority Review, and Breakthrough Therapy designations.
"Alzheimer's disease immeasurably incapacitates the lives of those who suffer from it and has devastating effects on their loved ones," said Billy Dunn, M.D., director of the Office of Neuroscience in the FDA's Center for Drug Evaluation and Research, in a press release on January 6, 2023.
"This treatment option is the latest therapy to target and affect the underlying disease process of Alzheimer's, instead of only treating the symptoms of the disease."
The results of a Phase 3 randomized, controlled clinical trial to confirm the drug's clinical benefit have recently been reported, and the agency anticipates receiving the data soon.
Alzheimer's disease is an irreversible, progressive brain disorder affecting more than 6.5 million Americans that slowly destroys memory and thinking skills and, eventually, the ability to carry out simple tasks.
While the specific causes of Alzheimer's are not fully known, it is characterized by changes in the brain—including amyloid beta plaques and neurofibrillary, or tau, tangles—that result in the loss of neurons and their connections.
These changes affect a person's ability to remember and think.
As of January 7, 2023, the FDA has not approved an Alzheimer's disease vaccine. However, there are several conducting clinical trials.
Disclosure: FDA and NIH announcements were manually curated.

Pfizer Inc. today announced that the U.S. Food and Drug Administration (FDA) accepted for priority review a supplemental Biologics License Application for its 20-valent pneumococcal conjugate vaccine candidate (20vPnC) for the prevention of invasive pneumococcal disease caused by the 20 Streptococcus pneumoniae (pneumococcus) serotypes contained in the vaccine in infants and children six weeks through 17 years of age.
And for preventing otitis media caused by seven of the 20 Streptococcus pneumoniae serotypes in the vaccine.
The Prescription Drug User Fee Act goal date for a decision by the FDA on the 20vPnC vaccine application is anticipated in April 2023.
“Today’s regulatory milestone further advances Pfizer’s commitment to the more than 20-year legacy of helping protect infants and children from invasive pneumococcal disease through conjugate vaccination,” said Annaliesa Anderson, Ph.D., SVP and Chief Scientific Officer, Vaccine Research and Development, Pfizer, in a press release on January 6, 2023.
“By offering the broadest serotype coverage by a pneumococcal conjugate vaccine against important serotypes causing pneumococcal disease in U.S. infants and children, 20vPnc, if approved, can help expand the protection for this vulnerable pediatric population.”
The FDA previously approved PREVNAR 20® (Pneumococcal 20-valent Conjugate Vaccine) on June 8, 2021, to prevent invasive disease and pneumonia caused by the 20 pneumococcus serotypes in the vaccine in adults ages 18 years and older.

The U.S. Centers for Disease Control and Prevention (CDC) today reported seasonal influenza activity remains high in the U.S. but is declining in most areas.
However, influenza-related fatalities have increased.
As of Week #51, the CDC disclosed 74 influenza-associated pediatric fatalities were reported during the 2022-2023 flu season. Unfortunately, 13 of those deaths were recently reported.
Furthermore, the CDC confirmed on January 6, 2023, that among the 2,380 pneumonia, influenza, and/or COVID-19 (PIC) fatalities last week, 303 were influenza-related.
The CDC continues every eligible person to get annual flu shots, which are generally available at clinics and pharmacies in the U.S.
"An annual flu shot is the best way to protect against influenza," CDC researchers wrote in today's weekly report.
"Vaccination helps prevent infection and can also prevent serious outcomes in people who still get sick with flu."