Search API
The U.S. Federal Aviation Administration (FAA) today announced at 0850 EST that normal air traffic operations are resuming gradually across the United States following an overnight outage to the FAA’s Notice to Air Missions (NOTAM) system.
The NOTAM provides safety information to flight crews when flying in the U.S.
Before commencing a flight, pilots are required to consult NOTAMs, which list potential adverse impacts on flights, from runway construction to the potential for icing.
Furthermore, the FAA is in the process of modernizing the NOTAM system to improve the delivery of safety-critical information to aviation stakeholders.
Earlier on January 11, 2023, the FAA confirmed departures were resuming at Newark Liberty and Atlanta Hartsfield-Jackson airports due to air traffic congestion in those areas.
The AP reported about 21,000 flights were scheduled for departure today.

Regeneron Pharmaceuticals, Inc. discretely revealed its intentions to expand its Anti-SARS-CoV-2 Monoclonal Antibody offerings at the J.P. Morgan Healthcare Conference on January 9, 2023.
Regeneron's presentation stated that in the U.S. alone, millions of immuno-compromised people will not adequately respond to vaccination.
And antibodies can be dosed prophylactically to prevent infection and severe COVID-19 disease.
On slide #27, the Company stated its 'Next-gen COVID antibody binds outside variable RBD and has demonstrated high neutralization activity against all known variants and lineages and disclosed it anticipates initiating the REGN14287 phase 3 clinical trial in 2023, pending regulatory discussions.
The NCT04425629 study was last updated on July 29, 2022.
Previously Regeneron's first-generation REGEN-COV monoclonal antibody combination (casirivimab and imdevimab) is U.S. Food and Drug Administration-approved and was a market leader in the U.S.
The U.S. NIH stated on December 1, 2022, vaccination remains the most effective way to prevent SARS-CoV-2 infection and should be considered the first line of prevention.
There are several new scientific developments regarding COVID-19 that might be useful to you for navigating the pandemic, wrote Katelyn Jetelina, Ph.D., an epidemiologist trying to make sense of this pandemic world.
All stem from different COVID-19 "story threads" I've written before. So, a quick round-up was posted by Jetelina at this substack link.
Separately, the U.S. CDC's Data Tracker publishes various information that is perpetually updated.
And the WHO publishes weekly epidemiological updates (Jan. 4, 2023) on the ongoing COVID-19 pandemic.

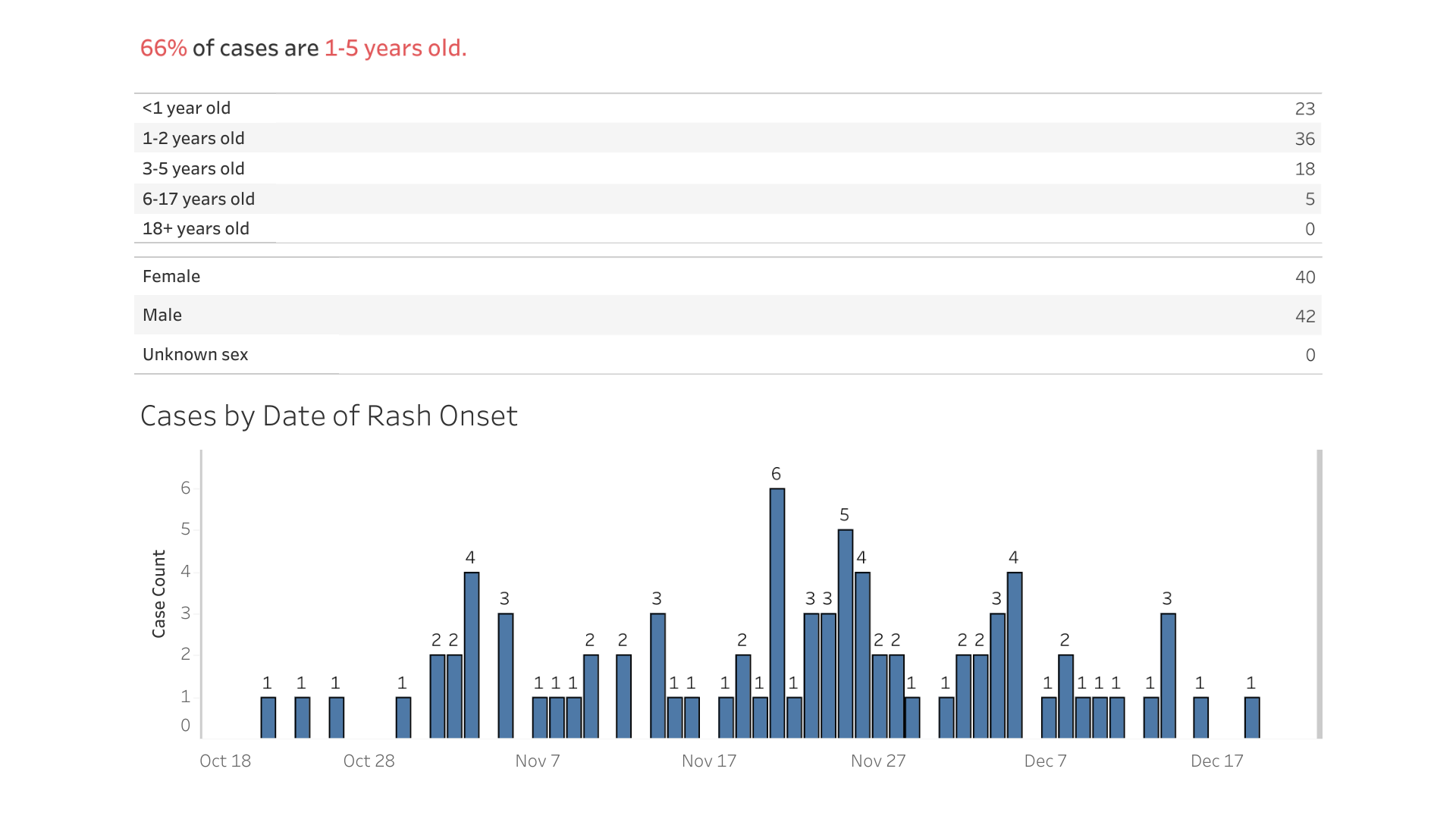
The City of Columbus, Ohio, reported yesterday new data regarding the measles outbreak in children that was initially identified in June 2022.
As of January 9, 2023, there have been 82 measles confirmations and 33 related hospitalizations.
About 75% of these children were unvaccinated before being infected with the measles virus.
Columbus's Health Commissioner, Dr. Mysheika W. Roberts, recently stated, "The end of an outbreak isn't declared until 42 days after the last infected person develops a rash."
"If you are interested in scheduling your child's MMR vaccine, you can contact your local healthcare provider or reach out to the Ohio Department of Health," reported SpectumNews1.
Measles is a vaccine-preventable disease, says the U.S. Centers for Disease Control and Prevention (CDC).
Additionally, since many measles cases are travel-related, the CDC suggests being fully immunized before traveling abroad, as several countries, such as India, have reported outbreaks in 2023.
In the U.S., measles vaccines are generally available at clinics and pharmacies.
Genexine Inc. recently announced updated results following the completion of its Phase 2 clinical study using GX-188E, its first-in-class proprietary DNA vaccine, in combination with KEYTRUDA® in 65 patients with HPV 16- and/or HPV 18- positive recurrent or metastatic advanced cervical cancer.
GX-188E is a therapeutic DNA vaccine that encodes the E6/E7 fusion protein of HPV subtypes 16 and 18 and is administered intramuscularly by electroporation.
On January 8, 2023, Genexine disclosed the final efficacy analysis of a phase 1/2 clinical trial showed an Objective Response Rate of 35% (21 of 60 patients), indicating that of the 60 patients with advanced cervical cancer, 21 patients saw either over 30% reduction in tumor size or complete remission.
Furthermore, cancer patients with a CPS<1 showed a response rate of 29.2%, while those with a CPS≥1 showed a response rate of 38.9%.
With a disease control rate of 57.0%, this combination therapy was effective in more than half of the patients.
The overall survival was 16.7 months which compares favorably to other agents that have been granted accelerated approval by U.S. Food and Drug Administration in 2nd line cervical cancer treatment.
Neil Warma, President and CEO of Genexine, commented in a press release on January 8, 2023, "... the results ... reinforce our belief that GX-188E could open up new treatment opportunities to all cervical cancer patients, especially PD-L1 negative patients who currently have limited options."
"We also appear to be extending patient survival beyond that of currently marketed drugs which should position us well as we move into larger Phase 3 studies to become a leader in the oncology DNA vaccine market."
Genexine management is presenting these data at the JP Morgan conference on January 9-13, 2023.
'The most important things you can do to help prevent cervical cancer are to get vaccinated against HPV, have regular screening tests, and go back to the doctor if your screening test results are not normal,' says the U.S. Centers for Disease Control and Prevention.
Disclosure: This announcement was curated for mobile readership and is not paid content.

SK bioscience today announced that it received a biologics license application approval for the 'SKYZoster™' shingles vaccine from the National Pharmaceutical Regulatory Agency in Malaysia.
SKYZoster is a live vaccine that attenuates the varicella-zoster virus and was initially approved in South Korea in 2017, then in Thailand in May 2020.
A recent Phase III clinical trial showed that SKYZoster is non-inferior compared to the control vaccine (Zostavax).
The cell-mediated immune response was also shown to be at an equal level, proving SKYZoster™ effectively induces immunogenicity against shingles, also known as herpes zoster.
Jaeyong Ahn, CEO of SK bioscience, commented in a press release on January 9, 2023, "It is encouraging that vaccines made by our technology are gradually expanding their influence in the global vaccine market where big pharmaceutical companies mainly dominate."
SKYZoster™ is steadily expanding its market position in South Korea. According to the IMS data, SKYZoster's market share in the third quarter of 2022 reached 56%.
Additionally, SK bioscience plans to submit for SKYZoster Pre-Qualification to the World Health Organization.
Shingles is a vaccine-preventable disease, says the U.S. Centers for Disease Control and Prevention (CDC).
About 33% of people in the U.S. will develop shingles in their lifetime.
If you've ever had chickenpox, you can get shingles. Even children can get shingles. And your risk of shingles increases with age says the CDC.
In the U.S., the Shingrix® vaccine is the market leader and is generally available at most clinics and pharmacies.
Other approved shingles vaccines and vaccine candidate news are posted at PrecisionVaccinations.com/Shingles.

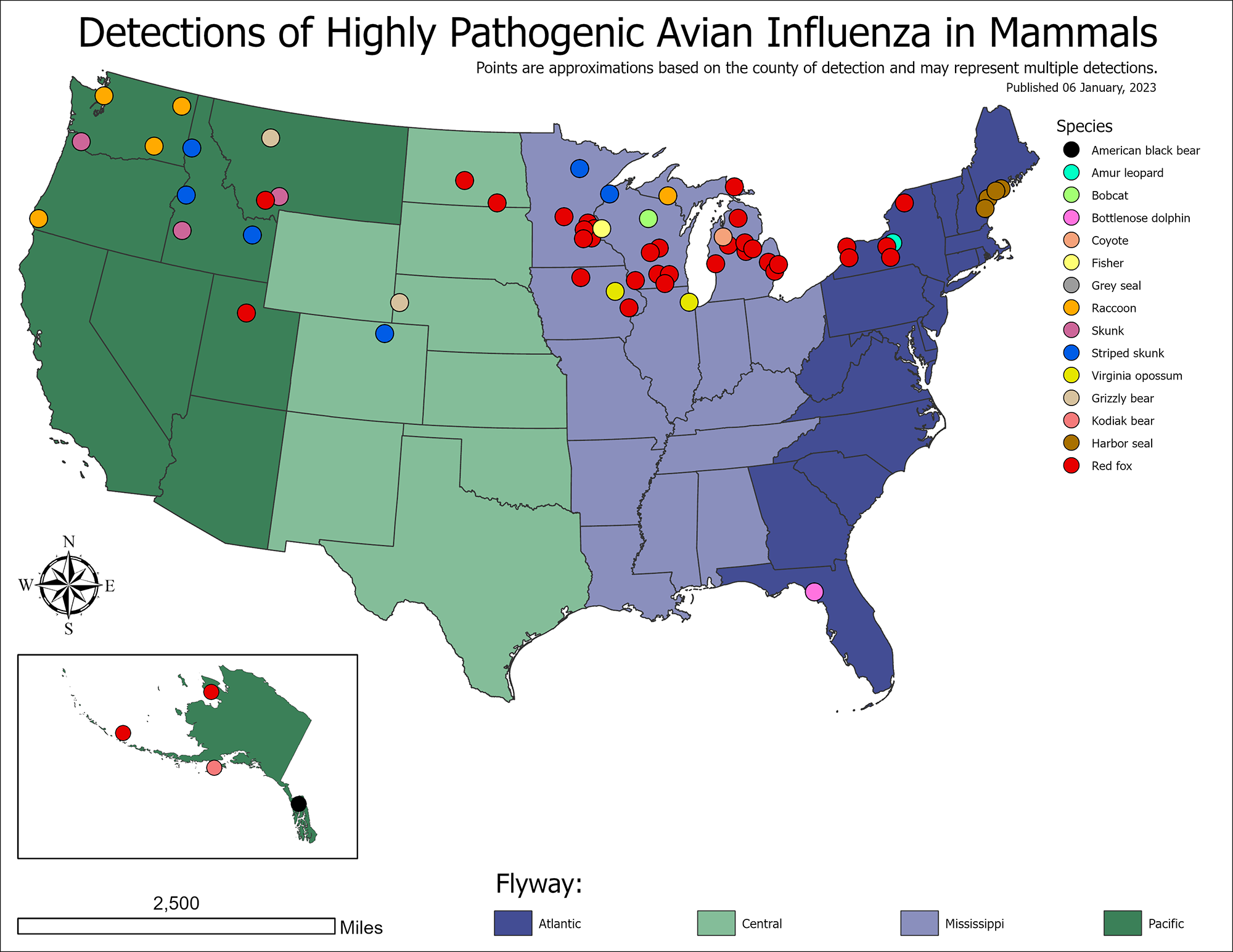
The US Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed today additional highly pathogenic avian influenza (HAPI) H5N1 detections in mammals.
The updated USDA listing as of January 9, 2023, includes skunks, bears, raccoons, and another red fox.
The USDA previously confirmed the Eurasian H5N1 strain first appeared in North America in January 2022.
On January 5, 2023, the StarHeral reported HAPI infections were the cause of death for four animals at the Riverside Discovery Center in Scottsbluff. The Nebraska zoo confirmed a cougar, bear, and two tigers had died.
The release indicates the animals ate local geese with HPAI in their systems.
The U.S. Centers for Disease Control and Prevention (CDC) says although bird flu (HAPI) viruses mainly infect and spread among wild migratory water birds and domestic poultry, some bird flu viruses can infect other animals as well.
While it’s unlikely people would become infected with bird flu viruses, it is possible, says the CDC.
Globally, six human influenza A H5N1 2.3.4.4b infections were reported last year, including a man working with birds in Colorado
Since April 2022, about 110 H5N1 detections in mammals were confirmed during 2022.
The USDA reported HAPI detections had affected 47 states and led to the loss of over 57.8 million birds as of January 4, 2023.
Additional HAPI (bird-flu) news is posted at PrecisionVaccinations.com/Avain.