Search API
An innovative dual-action cell therapy engineered to eliminate tumors, train the immune system to eradicate primary tumors, and prevent cancer recurrence is progressing.
Cancer vaccines are an active area of research for many labs, but this new approach is distinct.
Scientists in Boston are now harnessing a new way to turn cancer cells into potent, anti-cancer agents.
In the latest work led by Khalid Shah, MS, Ph.D. at Brigham and Women's Hospital, investigators have developed a new cell therapy approach to eliminate established tumors and induce long-term immunity, training the immune system to prevent cancer from recurring.
The team recently announced their dual-action, cancer-killing vaccine candidate in an advanced mouse model of the deadly brain cancer glioblastoma, with promising results.
"Our team has pursued a simple idea: to take cancer cells and transform them into cancer killers and vaccines," said the study's corresponding author Khalid Shah, MS, Ph.D., director of the Center for Stem Cell and Translational Immunotherapy (CSTI) and the vice chair of research in the Department of Neurosurgery at the Brigham and faculty at Harvard Medical School and Harvard Stem Cell Institute (HSCI), in a press release on January 4, 2023.
"Using gene engineering, we are repurposing cancer cells to develop a therapeutic that kills tumor cells and stimulates the immune system to destroy primary tumors and prevent cancer."
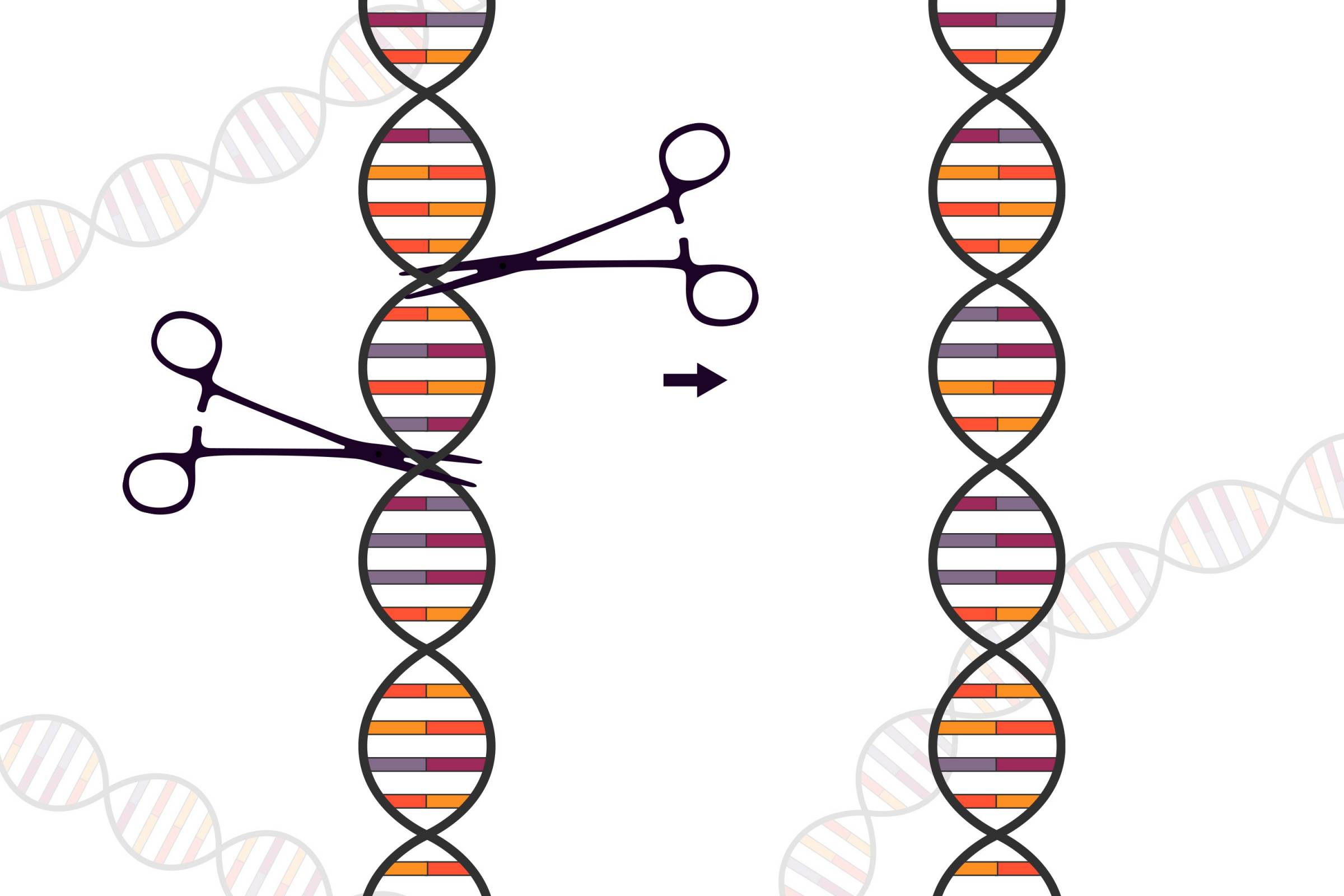
Instead of using inactivated tumor cells, the team repurposes living tumor cells, which possess an unusual feature.
Like homing pigeons returning to roost, living tumor cells will travel long distances across the brain to return to the site of their fellow tumor cells. Taking advantage of this unique property, Shah's team engineered living tumor cells using the gene-editing tool CRISPR-Cas9 and repurposed them to release tumor cell-killing agents.
In addition, the engineered tumor cells were designed to express factors that would make them easy for the immune system to spot, tag, and remember, priming the immune system for a long-term anti-tumor response.
The team tested their repurposed CRISPR-enhanced and reverse-engineered therapeutic tumor cells (ThTC) in different mice strains.
Shah's team also built a two-layered safety switch into the cancer cell, which, when activated, eradicates ThTCs if needed.
This dual-action cell therapy was found safe, applicable, and efficacious in these models, suggesting a roadmap toward therapy.
While further testing and development are needed, Shah's team specifically chose this model and used human cells to smooth the path of translating their findings for patient settings.
"Throughout all of the work that we do in the Center, even when it is highly technical, we never lose sight of the patient," added Shah.
"Our goal is to take an innovative but translatable approach to develop a therapeutic, cancer-killing vaccine that ultimately will have a lasting impact in medicine."
Shah and colleagues note that this therapeutic strategy applies to a broader range of solid tumors and that further investigations of its applications are warranted.
The study's findings are published in Science Translational Medicine on Jan. 4, 2023. Disclosures: Shah owns equity in and is a member of the Board of Directors of AMASA Therapeutics, a company developing stem cell-based therapies for cancer. This work was supported by the U.S. NIH (grant R01-NS121096).
According to recent data published by the U.S. Centers for Disease Control and Prevention (CDC), influenza vaccine distribution in the United States (U.S.) is approaching a similar amount compared to the last flu season.
Private manufacturers produce flu vaccines in the U.S., so yearly supply depends on manufacturer production.
As of December 31, 2022, the CDC confirmed 170.71 million influenza vaccines had been distributed.
During the 2021-2022 season, flu activity began to increase in November and remained elevated until mid-June, with two waves of influenza A(H3N2) virus activity occurring; the first peaked in late December 2021 and the second in April 2022.
Last flu season, vaccine manufacturers distributed 179.4 million doses of flu vaccine to the U.S. market as of February 25, 2022.
For the 2020-2021 flu season, 193.8 million doses were distributed in the U.S. as of February 26, 2021, the highest number of doses in a single flu season.
As each flu season ends, the CDC and vaccine producers update their vaccine offerings for the next season, highlighted at this link.
The CDC says getting an annual flu vaccine is the best way to protect yourself and your loved ones from influenza.
Flu vaccination is essential if you are at a higher risk of developing severe complications. When you get vaccinated, you reduce your risk of getting sick and possibly being hospitalized or dying from influenza.

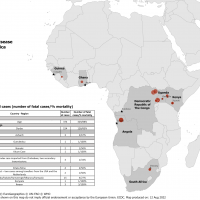
The U.S. Centers for Disease Control and Prevention (CDC) announced it joins the Republic of Uganda and the global public health community in marking the end of the Sudan Ebolavirus outbreak in Uganda.
Forty-two days, or two incubation periods, have passed since the last case of Sudan Ebola was reported in 2022, marking the official end of the fifth outbreak in Uganda, which had started in September.
In addition, the CDC confirmed entry screening and public health monitoring of travelers to the United States who have been in Uganda in the last 21 days would end on January 11, 2023.
The U.K. also launched airport screening for Ebola in November 2022.
“I commend the Government of Uganda, local health workers, and global public health partners who worked to end the country’s Ebola outbreak,” said CDC Director Rochelle P. Walensky, M.D., M.P.H., in a press release.
“I also want to thank the CDC staff on the front lines in Uganda and worldwide who worked countless hours to accelerate an end to the outbreak.”
The CDC confirmed it would continue supporting the Ugandan Ministry of Health in Ebola surveillance, infection prevention and control, and response activities to help ensure rapid detection and response to future cases and outbreaks.

The American Cancer Society (ACS) today released Cancer Statistics, 2023, the organization's annual report on cancer facts and trends.
According to the new ACS report, overall cancer mortality has dropped 33% since 1991, averting an estimated 3.8 million cancer deaths.
Based on ACS data, in 2023, there are projected to be 1,958,310 new cancer cases and 609,820 cancer deaths in the U.S.
Prostate cancer, which is the second leading cause of cancer death for men in the U.S., increased by 3% per year from 2014 through 2019 after two decades of decline.
Most concerning is that the diagnosis of advanced disease drove this increase.
Since 2011, the diagnosis of advanced-stage (regional- or distant-stage) prostate cancer has increased by 4% to 5% annually, and the proportion of men diagnosed with the distant-stage disease has doubled.
These findings underscore the importance of understanding and reducing this trend.
"The increasing percentage of men presenting with advanced prostate cancer, which is much more difficult to treat and often incurable, is highly discouraging," said Dr. Karen E. Knudsen, chief executive officer at the ACS, in a press release on January 12, 2023.
"In order to end cancer as we know it, for everyone, it is imperative for us to focus on cancers where trends for incidence and mortality are going in the wrong direction."
These major findings were published today in CA: A Cancer Journal for Clinicians, alongside its consumer-friendly companion, Cancer Facts & Figures 2023, available at https://www.cancer.org/research/cancer-facts-statistics.html.

The Ecuadoran Ministry of Public Health recently announced a nine-year-old girl in the province of Bolivar had been infected with the A-H5 strain of avian influenza after direct contact with infected birds.
This is Ecuador's first human infected with the avian flu strain, as reported by Xinhua on January 11, 2023. "So far, no other (bird flu) cases of human infection have been recorded since an outbreak began in 2022," said the ministry.
Globally, six other human influenza A H5N1 2.3.4.4b infections had been reported in China, Vietnam, the U.K., Spain (2), and Colorado during 2022-2023.
The Pan American Health Organization/WHO recommended on December 3, 2022, that the Member States strengthen coordination between sectors involved in alerting and responding to zoonotic events and implement the necessary measures to contain emerging pathogens that may put public health at risk.
Previously, South American countries, such as Belize, Columbia, Mexico, Peru, and Venezuela, confirmed bird flu cases in 2022.
In the U.S., the United States Department of Agriculture's Animal and Plant Health Inspection Service stated the Eurasian H5N1 strain first appeared in North America in January 2022, affecting 47 states, leading to the loss of over 57.8 million birds as of January 11, 2023.

Health Canada recently approved Enhertu™ for treating adult patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer who have received at least one prior line of chemotherapy in the metastatic setting or developed disease recurrence during or within six months of completing adjuvant chemotherapy.
Patients with hormone receptor-positive (HR+) breast cancer should have received at least one and be no longer considered eligible for endocrine therapy.1
Enhertu (trastuzumab deruxtecan) is a specifically engineered HER2-directed antibody-drug conjugate (ADC) jointly developed and commercialized by Daiichi Sankyo and AstraZeneca.
The approval by Health Canada on January 6, 2023, was based on the DESTINY-Breast04 Phase III trial results.
"Since the approval of HER2-targeted therapies in breast cancer more than twenty years ago, only patients with HER2-positive disease have been eligible for these therapies – leaving those with tumors with lower levels of HER2 expression with limited effective treatment options," said Dr. Jan-Willem Henning, Medical Oncologist, Tom Baker Cancer Centre, and Clinical Associate Professor, Cumming School of Medicine, University of Calgary, in a press release on January 12, 2023.
"The recent Health Canada approval of Enhertu in the HER2-low patient population is a significant milestone in the treatment of metastatic breast cancer and is the first anti-HER2 molecule to demonstrate efficacy outside of traditional HER2-positive breast cancer.
"Based on the promising data from the DESTINY-Breast04 trial, we're now able to differentiate levels of HER2 expression to redefine how we classify and treat metastatic breast cancer, providing additional patients with the opportunity to benefit from HER2-directed therapy."
In Canada, 10% of newly diagnosed breast cancers are metastatic; for those initially diagnosed with early-stage breast cancer, approximately 30% will progress to metastatic disease.
HER2 expression is currently defined as either positive or negative and is determined by an IHC test which estimates the amount of HER2 protein on a cancer cell, and/or an ISH test, which counts the copies of the HER2 gene in cancer cells.
However, approximately half of all breast cancers are HER2-low, and previously these patients had limited effective treatment options following progression on endocrine (hormone) therapy.
Health Canada reviewed and approved Enhertu for this indication under the Priority Review and Project Orbis FDA collaboration pathways seven months from filing, enabling the timely availability to bring this new treatment option to HER2-low breast cancer patients as quickly as possible.
Note: ENHERTU is U.S. FDA-approved for treating several types of cancer.