Search API
Global interest in developing vaccines against Neisseria gonorrhoeae (NG) has been sparked by the increasing threat of gonococcal antimicrobial resistance and the number of new infections.
And according to the U.S. Centers for Disease Control and Prevention (CDC), over 600,000 cases of gonorrhea were reported in 2020, making it the second most common sexually transmitted infection in the United States.
And the disease is known to be contracted repeatedly without apparently developing protective immunity.
To address this extensive health need, Intravacc today announced favorable preclinical data for Avacc 11®, the prophylactic intranasal gonorrhea candidate vaccine developed in partnership with Therapyx inc.
The results of the candidate, a proprietary outer membrane vesicle (OMV) platform-based gonorrhea vaccine combined with encapsulated IL-12, showed protection against subsequent infection with NG.
In this study, mice were vaccinated via the intranasal route, and the results of this intranasal study were similar to the intravaginal vaccination route. Intranasal immunization resulted in high serum IgG, salivary IgA, and vaginal IgG and IgA anti-gonococcal antibodies when OMVs were administered with IL-12 ms.
The serum IgG and salivary IgA antibodies induced in male mice were similar to the response induced in female mice.
Gamma interferon (IFN-g) production by CD4 T cells from iliac lymph nodes was elevated after vaccination intranasally or intravaginally.
Female mice immunized with OMVs plus IL-12 ms by either route resisted challenge with NG to an equal extent, and resistance generated by intranasal immunization extended to heterologous strains of NG.
These results were published in the peer-reviewed journal MSphere of the American Society of Microbiology.
Dr. Jan Groen, Intravacc's CEO, commented in a press release on January 16, 2023, "Together with our partner Therapyx, we are very pleased with the preclinical data of the intranasal candidate vaccine Avacc 11®."
"This intranasal gonococcal vaccine is more suitable to fight gonorrhea infections, which are becoming increasingly resistant to antibiotic treatments."
In October 2022, Intravacc was awarded a $14.6 Million U.S. NIH/NIAID contract to develop this intranasal candidate gonorrhea vaccine further.
For the development of vaccines, Intravacc has designed and developed a platform based on outer membrane vesicles, spherical particles with intrinsic immune-stimulating properties.
The OMVs can be designed with immunogenic peptides and/or proteins that stimulate effective adaptive immunity.
The OMV carrier has been optimized to induce a more effective immune response against these newly introduced antigens.
Intravacc has also developed genetic tools to increase the yield of the OMVs, reduce toxicity and achieve the desired antigenic composition.
Intravacc's OMV platform is scalable and allows rapid and efficient modification of the antigen composition, either through genetic modification of the bacterial host or by associating antigens with stored OMVs.
As of January 2023, there is no effective gonorrhea vaccine available in the U.S.

Since influenza is an airborne virus, efforts to avoid catching the flu depend on your proximity to an infectious (sneezing) person. While this logic is simple to say, what reports can a person trust to make virus avoidance decisions in 2023?
According to the World Health Organization (WHO) Influenza Update N° 436, most countries of North America indicate influenza activity decreased while others were stable or continued to increase.
The WHO finding was supported by the recent U.S. Centers for Disease Control and Prevention (CDC) report issued on January 13, 2023, that stated seasonal influenza activity continues but is declining in most areas, with specimens testing positive for influenza in clinical laboratories decreasing in all regions.
Elsewhere around the globe, the WHO reported:
In Europe, overall influenza activity continued to increase, with influenza positivity from sentinel sites remaining above the epidemic threshold at the regional level.
In central Asia, influenza activity increased with influenza A(H1N1)pdm09 viruses predominant, followed by influenza B viruses.
In Northern Africa, influenza detections increased among reporting countries with all seasonal subtypes detected. And influenza activity remained low in tropical Africa, with detections of all seasonal influenza subtypes reported. However, activity increased in some countries in Eastern Africa.
In Western Asia, influenza activity decreased overall with all seasonal influenza subtypes detected, though increased activity was reported in some countries.
In East Asia, influenza activity of predominantly influenza A(H3N2) viruses remained low overall among reporting countries but with increases reported in Mongolia and the Republic of Korea.
In the Caribbean and Central American countries, influenza activity of predominantly influenza A(H3N2) viruses decreased overall but remained elevated in Mexico.
In the tropical countries of South America, influenza detections were generally low. However, influenza positivity was at a moderate level in Ecuador.
In the temperate zones of the southern hemisphere, influenza activity decreased in Argentina and Chile to low levels and remained low elsewhere.
In Southern Asia, influenza activity remained low, mainly due to decreased activity reported in the Islamic Republic of Iran.
In South-East Asia, detections of predominantly influenza B remained elevated due to continued detections reported in Malaysia.
Before visiting these areas, the CDC suggests speaking with a doctor, nurse, or pharmacist to ensure you are appropriately protected from influenza.
In the U.S., access to flu shots remains abundant, as over 157 million vaccines have already been distributed and are generally available at most clinics and pharmacies.
As the new year began a few weeks ago, various scientists are focused on developing Human Immunodeficiency Virus (HIV) vaccines. And vaccine development has accelerated in 2023 with candidates utilizing innovative technologies such as mRNA.
Vaccines work by inducing the immune system to make antibodies that can neutralize a particular pathogen.
But doing so for HIV has been challenging because there are countless variants worldwide, wrote the U.S. National Institutes of Health (NIH) on December 13, 2022.
This challenge is why mRNA vaccines may become the solution.
Encouraging news was announced in 2022 when Moderna Inc., a global leader in mRNA vaccines, confirmed it was participating in the NIH's HVTN 302 study that examines the safety and immune responses of BG505 MD39.3 mRNA, BG505 MD39.3 gp151 mRNA, and BG505 MD39.3 gp151 CD4KO vaccines.
Each of Moderna's vaccine candidates are designed to present the spike protein found on the surface of HIV that facilitates entry into human cells and encodes for different but highly related stabilized proteins.
While this early-stage, Phase 1 clinical trial was updated on October 3, 2022, it could be years from achieving U.S. Food and Drug Administration (FDA) approval.
As of January 15, 2023, the FDA had not approved any HIV prevention vaccine for use by people.
Unfortunately, the AIDS epidemic continues to impact people everywhere, specifically in Africa.
About 38 million people worldwide are living with HIV, and about 70% of them live in Africa.
However, over 28 million people were accessing antiretroviral therapy in 2022, a significant increase from 7.8 million in 2010.
And in 2023, these people have expanded treatment options.
Gilead Sciences, Inc. announced on December 22, 2022, that the FDA approved Sunlenca® for treating HIV-1 infections in heavily treatment-experienced adults with multi-drug resistant HIV-1 infection.
And previously, the U.S. FDA-approved Apretude for use by at-risk adults and adolescents weighing at least 35 kilograms for pre-exposure prophylaxis to reduce the risk of sexually acquired HIV on December 20, 2021.
Anthony S. Fauci, M.D., former director at the U.S. National Institute of Allergy and Infectious Diseases, recently commented about HIV vaccine development efforts in an Emerging and Reemerging Infectious Diseases Perspective: It Ain't Over Till It's Over…but It's Never Over.

Each year, the American Cancer Society (ACS) estimates the number of new cancer cases and related fatalities in the United States (U.S.). Cancer is the second leading cause of death in the United States, exceeded only by heart disease.
On January 12, 2022, the ACS published a study with good news regarding cervical cancer.
This new ACS study highlights a 65% decrease in cervical cancer incidence from 2012 through 2019 among women in their early 20s.
This is the first cohort to receive the human papillomavirus vaccine (HPV) early in life, which foreshadows steep reductions in the burden of cancer.
This is essential news since there were 12,795 new cases of cervical cancer reported among women, and 4,152 women died of this cancer n 2019.
Surprisingly, cervical cancer herd immunity has also been identified in the U.S. based on data from the National Health Examination Survey from 2003 through 2018.
This data shows reductions in HPV-16 and HPV-18 infection among sexually active females aged 14–24 years, of 90% among those vaccinated and 74% among those unvaccinated.
Sweden was the first to report a population-level reduction in invasive cervical cancer incidence of 78% among women vaccinated before the age of 17 in 2020.
Shortly after that, an 87% reduction in cervical cancer and a 97% reduction in grade 3 cervical intraepithelial neoplasia was demonstrated among women aged 20–29 years who were vaccinated at ages 12 to 13 years in England.
Although up-to-date (three-dose) HPV vaccination coverage in the U.S. has lagged behind other countries, accumulating evidence suggests that a single dose offers substantial protection and may even be preferable in low-income, high-burden populations.
In April 2022, the World Health Organization's Strategic Advisory Group of Experts on Immunization endorsed single-dose vaccination among girls aged 9–14 to address the global shortfall and optimize cancer prevention.
In 2021, 79% of adolescent girls in the United States had received at least one dose, and 64% were fully up to date.
HPV vaccines are generally available at clinics and pharmacies in the U.S.
The State of Kentucky recently announced a panel of the Sixth Circuit U.S. Court of Appeals affirmed a lower court's ruling that said vaccine mandates were unconstitutional. The mandate requires workers contracting with the federal government to wear face masks and be vaccinated for COVID-19.
A federal judge in Louisville, Kentucky, blocked the U.S. government's rule in November 2021 for Kentucky, Tennessee, and Ohio.
"The Sixth Circuit's decision is a resounding victory against federal overreach into the personal medical decisions of Kentuckians," Kentucky Attorney General Daniel Cameron said in a statement on January 12, 2023.
The Fifth Circuit U.S. Court of Appeals issued a similar ruling for Indiana, Louisiana, and Mississippi in December 2022.
The U.S. Centers for Disease Control and Prevention (CDC) says certain workers are at risk for exposure to severe and sometimes fatal diseases.
If you work directly with patients or handle material that could spread infection, you should get appropriate vaccines to reduce the chance that you will get or spread vaccine-preventable diseases.
And protect yourself, your patients, and your family members by ensuring you are up-to-date with recommended vaccines, including COVID-19, says the CDC.

Earlier today, WHO Director-General Dr. Tedros Adhanom Ghebreyesus spoke to the director of China’s National Health Commission, Minister Ma Xiaowei.
The WHO confirmed in a media statement on January 14, 2023; it appreciates this telephonic meeting and the public release of information about the pandemic in the country.
Chinese officials provided information to WHO and in a press conference on various topics, including outpatient clinics, hospitalizations, patients requiring emergency treatment and critical care, and hospital deaths related to COVID-19 infection.
WHO is analyzing this information, which covers early December 2022 to January 12, 2023, and allows for a better understanding of the epidemiological situation and the impact of this wave in China.
The overall epidemiology—reflecting a rapid and intense wave of disease caused by known sub-variants of Omicron with higher clinical impact on older people and those with underlying conditions—is similar to waves of infection experienced by other countries, as is the increased pressure on health services.
Nevertheless, the reported data indicate a decline in case numbers, hospitalizations, and those requiring critical care.
The WHO has requested a more detailed breakdown of data by province over time.
While the Chinese Center for Disease Control and Prevention has earlier reported that Omicron sublineages BA.5.2 and BF.7 are currently circulating, WHO continues to ask that other sequences be shared with open access databases such as GISAID for deeper phylogenetic analyses and continued collaboration with technical groups working on virus evolution, clinical care, and beyond.
The WHO stated it would continue to work with China, providing technical advice and support and analyzing the situation.
Note: The unedited, complete media statement is available at this WHO link.

The World Health Organization (WHO) today called on countries to take urgent measures against measles outbreaks which are on the rise, with nearly nine million children having missed vaccination against the killer disease in the last two years.
Measles outbreaks are declared when the number of cases reported in an area is higher than expected.
"The strong political commitment, determination, focused and concerted efforts, and community support that marked our efforts for polio elimination are now urgently needed to stop and prevent measles outbreaks and accelerate efforts to eliminate the disease," said Dr. Poonam Khetrapal Singh, Regional Director, WHO South-East Asia, in a media release on January 13, 2023.
Measles and rubella elimination is a flagship priority program of the WHO South-East Asia Region. Both are vaccine-preventable.
Between 2014 and 2021, the Region recorded a 73% reduction in measles deaths and a 64% reduction in measles cases.
Five of the 11 countries in the South-East Asia Region – Bhutan, DPR Korea, Maldives, Sri Lanka, and Timor-Leste - have eliminated measles, and two countries - Maldives and Sri Lanka - have eliminated rubella as well.
In addition, governments have been administering two doses of measles vaccines as part of their childhood immunization program, and some are rolling out mass vaccination campaigns.
However, as the pandemic arrived, measles vaccination coverage, which had reached an all-time high of 94% coverage for the first dose and 83% coverage for the second dose by 2019, declined to 86% and 78%, respectively, in 2021.
This inaction left about nine million children unvaccinated for measles.
And around 5.3 million children are partially vaccinated against this highly infectious disease.
"The decline in vaccine coverage, and interruptions and delays in immunization and surveillance activities due to the pandemic, leaves the Region susceptible to large outbreaks and off track for the 2023 target of measles and rubella elimination," the Regional Director added.
"All countries have been taking several initiatives to revive and resume childhood immunization coverage and surveillance activities that were affected by the COVID-19 pandemic," Dr. Khetrapal Singh concluded.
The U.S. CDC says measles is highly contagious, with about 90% of people who are not protected will become following infected exposure to the measles virus.
The CDC's Top 10 Measles Outbreak Country list was updated on January 13, 2023.
International travelers can check CDC's Travel Health Notices to search for measles or other issues that may affect travelers' health. During 2022, the CDC issued various measles-related travel alerts.
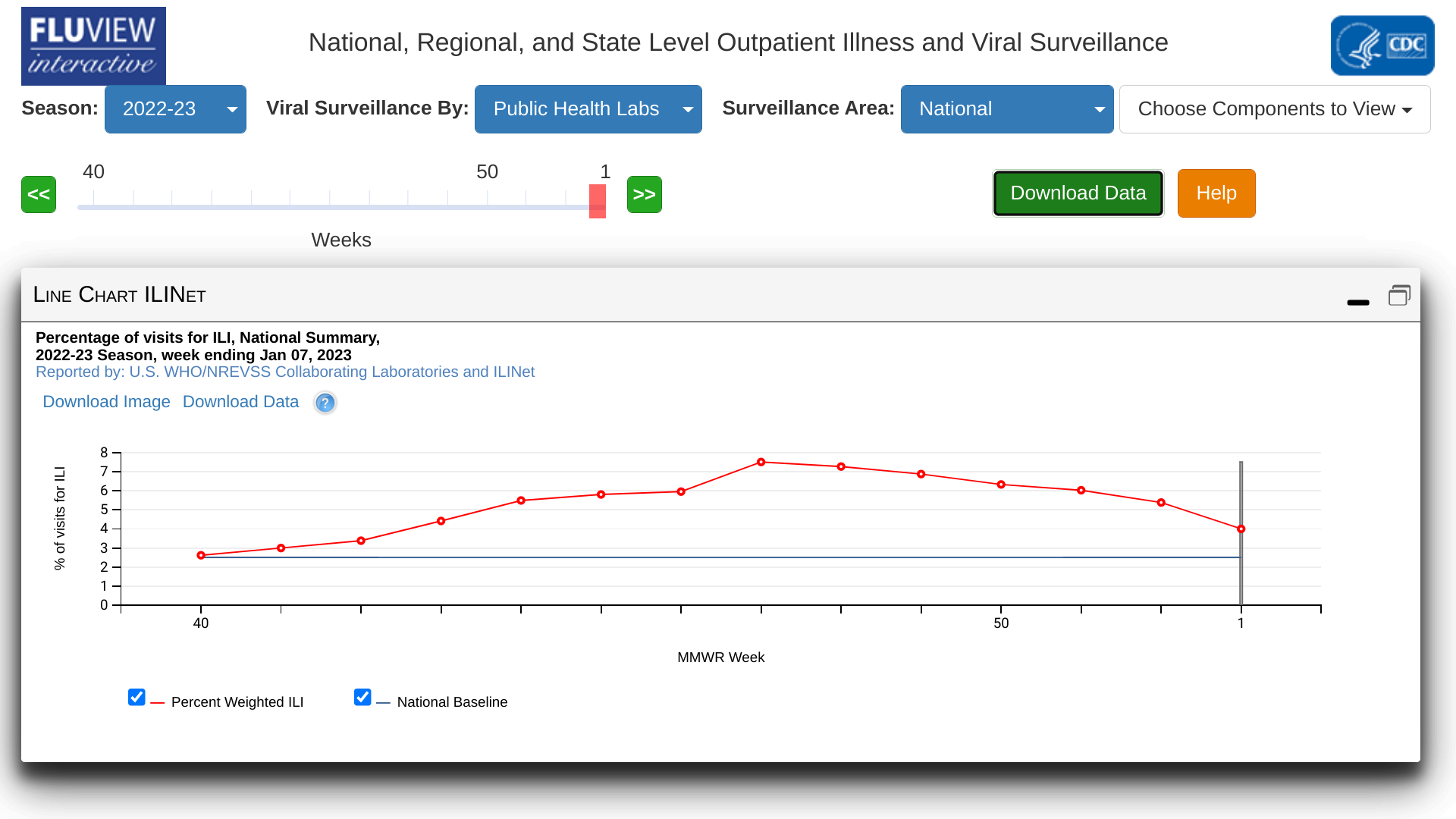
The U.S. Centers for Disease Control and Prevention (CDC) reported today the percentage of specimens testing positive for influenza in clinical laboratories across the nation declined compared to the previous week.
As of January 13, 2023, the CDC's Week #1 Influenza Surveillance Report confirmed the number of flu hospital admissions reported in the HHS Protect system decreased compared to week #52 (Dec. 2022).
And the cumulative hospitalization rate in the FluSurv-NET system was lower than end-of-season hospitalization rates for most pre-pandemic seasons going back to 2010-2011.
The good news reported by the CDC this week was the majority of influenza viruses tested are in the same genetic subclade as and antigenically similar to the influenza viruses included in this season's influenza vaccines.
Additional good news highlighted that over 170 million flu shots had been distributed in the U.S. this flu season, eliminating access concerns.
Most clinics and pharmacies have an ample supply of various flu shots approved for the 2022-2023 season.
The CDC says ab annual flu vaccine is the best way to protect against the health risks posed by an influenza infection.
And vaccination helps prevent infection and serious outcomes in people who get vaccinated but still get sick with the flu.
This CDC advice is very relevant for children, as 79 influenza-associated pediatric fatalities have been reported during the 2022-2023 season.
Furthermore, the CDC continues to recommend that everyone ages six months and older get an annual flu vaccine as long as flu activity continues.