Search API
Moderna, Inc. announced positive topline data from its ConquerRSV Phase 3 pivotal efficacy trial of mRNA-1345, an mRNA vaccine candidate targeting respiratory syncytial virus (RSV) in older adults.
Following review by an independent Data and Safety Monitoring Board (DSMB), the primary efficacy endpoints have been met, including vaccine efficacy (VE) of 83.7% (95.88% CI: 66.1%, 92.2%; p<0.0001) against RSV-associated lower respiratory tract disease as defined by two or more symptoms.
Based on these results, Moderna stated on January 17, 2023, it intends to submit for regulatory approval for mRNA-1345 in the first half of 2023.
"RSV significantly affects the health of older and high-risk adults, particularly those with comorbidities," commented Abdullah Baqui, a principal investigator for the study sites in Bangladesh and Professor, Department of International Health, Director, International Center for Maternal and Newborn Health, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, in a related press release.
"This trial will help to understand the role of severe acute respiratory infections in older adult populations and inform the future implementation of vaccines in adults in lower-resource areas."
A concurrent review of available safety data was also conducted by the DSMB.
mRNA-1345 was well tolerated, with no safety concerns identified. To date, most solicited adverse reactions were mild or moderate, and the most commonly reported solicited adverse reactions in the mRNA-1345 group were injection site pain, fatigue, headache, myalgia, and arthralgia.
The overall rate of severe (Grade 3 or greater) solicited systemic adverse reactions was 4.0% for mRNA-1345 and 2.8% for placebo.
The overall rate of Grade 3 or greater solicited local adverse reactions was 3.2% for mRNA-1345 and 1.7% for placebo.
The study is ongoing, and an updated analysis of safety and tolerability will be provided at the time of regulatory submission.

Jefferson County Public Schools (JCPS) in Kentucky announced yesterday it would begin conducting measles vaccination clinics at local schools for about 10,000 unvaccinated students.
JCPS distributed notices to Louisville-area families whose school records show their students need to be protected against this vaccine-preventable disease.
Measles is highly contagious and often results in the hospitalization of those who contract the virus.
Beginning on January 17, 2023, JCPS students will be able to receive a Measles, Mumps, and Rubella (MMR) vaccine, as well as the COVID-19 and flu vaccines.
'We urge parents and guardians to ensure students are current with all required vaccines, including the MMR.
The U.S. CDC and the Kentucky Department of Public Health urge families to be aware of an ongoing measles outbreak near Columbus, Ohio.
As of January 17, 2023, the Columbus Health Department had confirmed 85 children had been infected with measles since June 2022. Most of these unvaccinated children are under ten years of age.
Previously, the CDC confirmed (118) measles patients in six jurisdictions in 2022. In 2021, a total of 49 measles cases were reported by five jurisdictions.
The CDC's top ten global measles outbreaks as of January 13, 2023, were led by India, with 12,271 cases, and Yemen, with 7,538.
In the U.S., various MMR vaccines are available at most clinics and pharmacies in 2023.

During today's World Economic Forum Annual Meeting in Switzerland, Dr. Tedros Adhanom Ghebreyesus and others discuss what mechanisms can accelerate the development and deploy safe and effective tuberculosis (TB) vaccines.
The 'Ending Tuberculosis: How Do We Get There' live discussion is at this link.
While no new TB vaccine has been licensed in 100 years, the prospects for novel effective TB vaccines have improved recently, with at least 16 vaccine candidates under development.
According to the World Health Organization (WHO) 2022 Global TB report, more than 10 million people fell ill from TB, and 1.6 million died.
The WHO confirmed effective vaccines would undoubtedly be the best solution to prevent and potentially eradicate TB.
A recent WHO-commissioned study, An investment case for new TB vaccines, estimates that, over 25 years, a vaccine that is 50% effective in preventing disease among adolescents and adults could avert up to 76 million new TB cases and US$ 6.5 billion in costs.
The current century-old bacille Calmette-Guérin (BCG) vaccine continues offering disease protection.
According to a recent study funded by the U.S. NIH, BCG vaccination at birth effectively prevents TB in young children.
And on August 15, 2022, research published by Cell Reports Medicine suggested BCG protection against infectious diseases and vaccine efficacy takes 1-2 years to manifest, but the protection may last decades.
In the U.S., the BCG vaccine is a limited distributed product. BCG is only considered for people who meet specific criteria and are in consultation with a TB expert., says the U.S. CDC.

The Coalition for Epidemic Preparedness Innovations (CEPI) and Vaxxas today announced an agreement to advance the development of needle-free vaccine-patch delivery technology in a project that could end the need for frozen storage of mRNA vaccines.
CEPI confirmed on January 17, 2023, it would provide up to $4.3 million (AUD6.4 million) for preclinical testing of Vaxxas' platform, a needle-free, high-density microarray patch (HD-MAP) to assess its stability, safety, and immunogenicity.
And to evaluate its potential as a rapid-response technology for heat-stable, dried-formulation mRNA vaccines.
In addition to a Phase I clinical study of a COVID-19 vaccine candidate patch, Vaxxas is performing demonstration work in preparation for clinical evaluation under contract with the U.S. government on pandemic vaccination solutions.
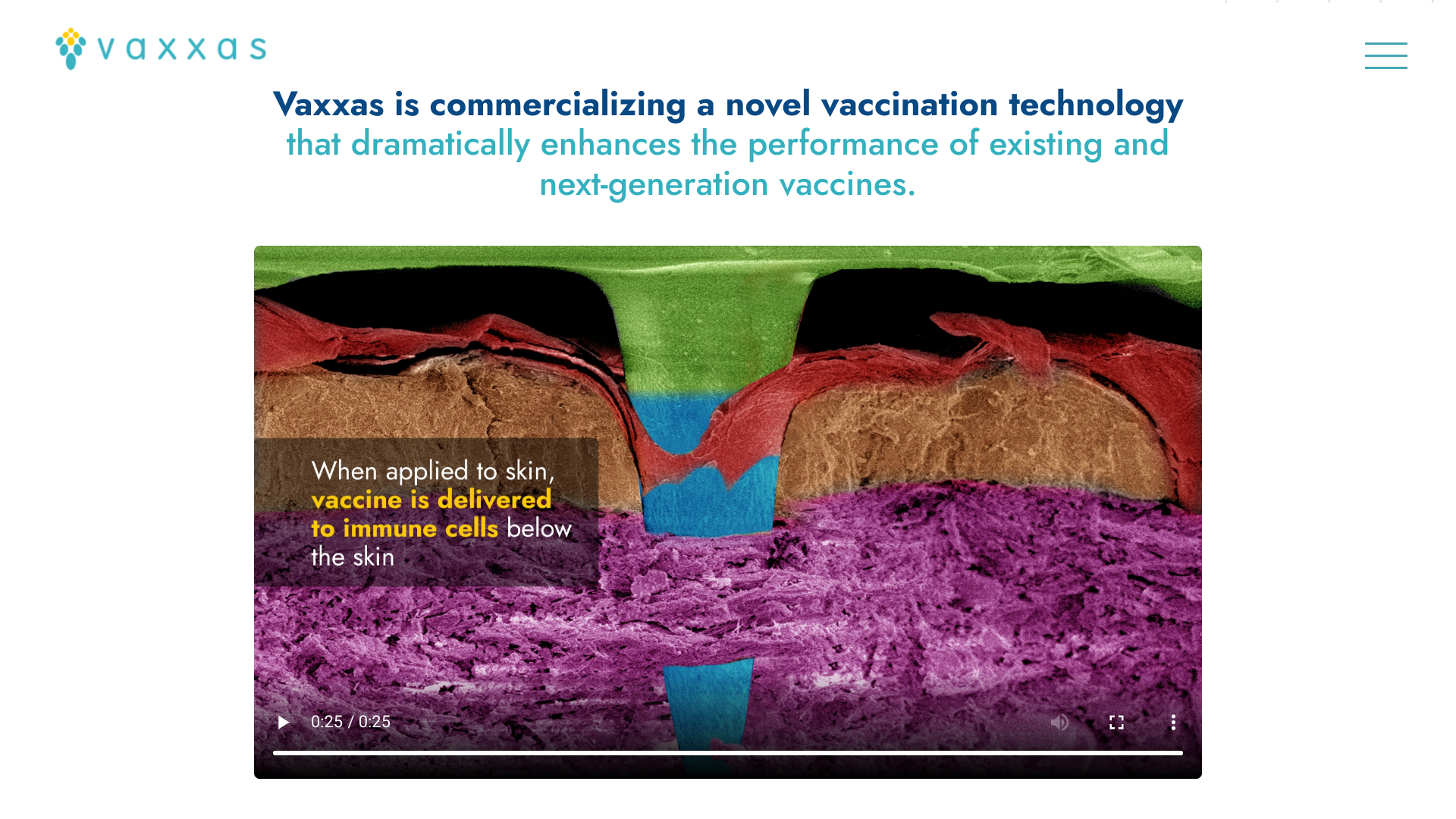
HD-MAPs comprise thousands of microscopic points attached to a small patch.
Each of these micro-projections contains a tiny dose of vaccine in a dried formulation. When applied to the skin, the patch delivers the vaccine to the abundant immune cells immediately below the skin surface.
HD-MAP vaccine delivery offers many advantages over more traditional vaccine administration methods.
For example, the dried form of the vaccine is more stable at higher temperatures than vaccines in liquid formulations.
Vaxxas' HD-MAPs have proven safe and tolerable in hundreds of trial participants and have been shown to induce equal or greater immune responses to injected vaccines at lower doses.
Compared with needle and syringe systems, they are also much easier to administer and are likely to have greater acceptability.
Ultimately, HD-MAP patches could enable a future in which vaccine patches could be mailed directly to peoples' homes, workplaces, and schools, avoiding the delay and inconvenience of traditional needle-and-syringe vaccine scheduling and administration.
David Hoey, Vaxxas's CEO, commented in a related press release, "Earning this significant funding from one of the world leaders in vaccine development is a great honor and validates the benefits offered by Vaxxas' HD-MAP vaccine platform in the fight against global epidemic and pandemic threats."
"In addition to providing an opportunity to get life-saving vaccines for infectious diseases that have a disproportionate impact on the most vulnerable populations around the world, the advanced development of HD-MAP delivery of mRNA vaccines could also prove very beneficial for the development of Vaxxas' internal pipeline across several diseases, including Covid-19."
Vaxxas' core technology was initially developed at The University of Queensland in Australia. The private company was established as a start-up in 2011.
On December 5, 2022, Vaxxas announced it completed a financing round that raised US$23 million in new funds.
CEPI is an innovative partnership between public, private, philanthropic, and civil organizations, launched in 2017, to develop vaccines against future epidemics. Its mission is to accelerate the development of vaccines and other biologic countermeasures against epidemic and pandemic threats so they can be accessible to all needy people.
Pfizer Inc. today announced that it has significantly expanded its commitment to An Accord for a Healthier World to offer the full portfolio of medicines and vaccines for which it has global rights on a not-for-profit basis to enable greater health for 1.2 billion people living in 45 lower-income countries.
To better align with disease burden and unmet patient needs in these countries, Pfizer is expanding its offering under the Accord to include off-patent products, bringing the total offering to around 500 products.
Launched in May 2022, the Accord is a transformative initiative focused on reducing health inequities.
The Accord initially included a commitment from Pfizer for access to all its patented medicines and vaccines available in the U.S. or European Union on a not-for-profit basis to 45 lower-income countries.
The Accord portfolio offering now includes chemotherapies and oral cancer treatments that can potentially treat nearly one million new cancer cases in Accord countries each year.
It also includes a wide range of antibiotics that can help to address the rising morbidity, mortality, and costs associated with antimicrobial resistance.
As Pfizer launches new medicines and vaccines, those products will also be included in the Accord portfolio on a not-for-profit basis.
“We launched the Accord to help reduce the glaring health equity gap that exists in our world. Our hope is to empower country governments and co-create solutions with them and other multi-sector partners to break down many of the system-level barriers to better health. In the months since the Accord’s launch, we have heard resoundingly from these leaders that access to a broader and more immediate scope of consistent, high-quality products is needed for meaningful and sustainable transformation. We believe this expansion of our product offering, combined with continued efforts to help address the barriers that limit or prevent access, will help us to achieve and even expedite our vision of a world where all people have access to the medicines and vaccines they need to live longer and healthier lives,” said Pfizer Chairman and CEO Albert Bourla, in a press release on January 17, 2023.
Alongside governments and multi-sector partners, Pfizer is working to co-create scalable solutions that help address systemic barriers to better health focused on finding faster, more efficient pathways for the supply of medicines and vaccines as well as strengthening the resources, capabilities, and platforms that can enable quick and more sustainable access to those medicines.
This includes technical expertise, training, diagnostic capacity, innovative financing, and more.
“The Accord is an important step toward sustainable health security for Rwanda and the broader continent. The expanded portfolio offering and public health system strengthening efforts will further enhance our progress and offer valuable support to key national health initiatives that lead to positive health outcomes,” commented His Excellency Paul Kagame, President of Rwanda.
Further details about An Accord for a Healthier World are available at Pfizer.com/Accord.

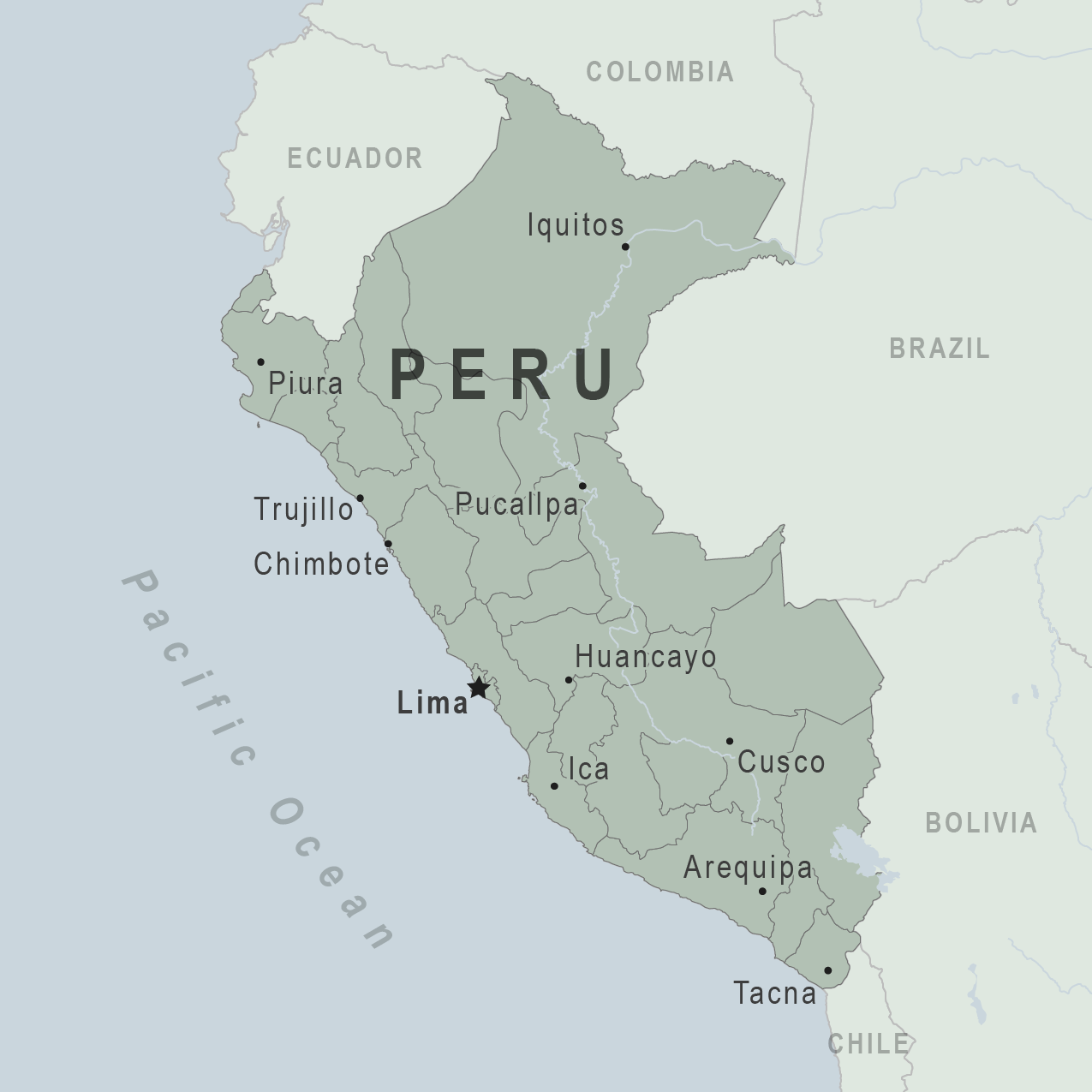
The U.S. Embassy in Peru recently confirmed the Government extended a 30-day State of Emergency in selected areas of Peru starting January 15, 2023. The affected areas include the departments of Cusco, Puno, Lima, and the province of Callao.
The province of Andahuaylas in the department of Apurimac, the provinces of Tambopata and Tahuamanu in the department of Madre de Dios, the district of Torata, and the province of Mariscal Nieto in the department of Moquegua are included.
Some national highways are affected, including the Pan-American Highway as well as the Apurimac-Cusco-Arequipa roadway.
For additional details, please see the complete Supreme Decree.
The Embassy suggests U.S. citizens in Peru avoid crowds and demonstrations, comply with instructions from local authorities, and enroll in STEP to receive alerts and messaging from the U.S. Embassy in Lima.
And for in-country assistance, visit the U.S. Embassy in Lima, Peru, at Avenida La Encalada cdra, 17 s/n, Santiago de Surco 15023, Lima , or contact +51-1-618-2000 and [email protected].
From a health perspective, the U.S. CDC says to check the vaccines and medicines list and visit your healthcare provider at least a month before your trip to Peru to get vaccines or medicines you may need.
The U. S. Centers for Disease Control and Prevention (CDC) recently announced it joined the Republic of Uganda in marking the end of the fifth Sudan Ebolavirus outbreak in Uganda.
The last Sudan Ebola outbreak in Uganda was in 2012.
In addition, entry screening and public health monitoring of travelers to the U.S. who have been in Uganda in the last 21 days ended on January 11, 2023.
“I commend the Government of Uganda, local health workers, and global public health partners who worked to end the country’s Ebola outbreak,” said CDC Director Rochelle P. Walensky, M.D., M.P.H., in a media statement.
The CDC confirmed it would continue supporting the Ugandan Ministry of Health in continuing surveillance, infection prevention and control, and response activities to help ensure rapid detection and response to future cases and outbreaks.
Since this outbreak declaration in September 2022, there were 164 cases with a case-fatality ratio was 47%.
Furthermore, three vaccine candidates launched human clinical trials for this type of Ebola in December 2022.