Search API
According to research published in The Lancet Infectious Diseases by researchers at the University of Maryland School of Medicine (UMSOM), a monoclonal antibody (mAbs) treatment conferred protection in people against P falciparum (malaria) at low doses
Published on January 25, 2023, this phase 1 clinical study found the mAbs CIS43LS protected 18 (82%) of 22 participants who received a dose. In addition, no participants developed parasitemia following dosing at 5 mg/kg intravenously or subcutaneously or at 10 mg/kg intravenously or subcutaneously.
All six control and four of seven participants dosed at 1 mg/kg intravenously developed parasitemia after controlled human malaria infection.
"The study demonstrates the feasibility of using mAbs therapies to help prevent malarial infection and holds promise for deployment to places where the disease is endemic," said Kirsten Lyke, MD, at UMSOM, in a related press release.
"This may allow us to revisit malaria eradication efforts."
According to the World Health Organization (WHO), malaria is a vaccine-preventable disease caused by a parasite.
Vaccines like Mosquirix™ (RTS,S), and R21/Matrix-M™ have been reported to be effective at preventing disease in Africa and India.
As of February 11, 2023, the U.S. Food and Drug Administration (FDA) had not approved a malaria vaccine.
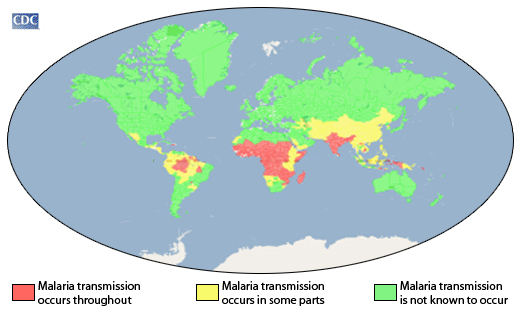
According to the 2021 World Malaria Report, about half the world's population lives in areas at risk of malaria transmission.
There were 241 million malaria cases and 627,000 deaths reported worldwide in 2020 alone, a 12% increase from 2019.
However, outbreaks of locally transmitted malaria cases in the U.S. have been limited and relatively isolated. The FDA has approved Artesunate to treat severe malaria in adult and pediatric patients.
Malaria outbreak news is posted at Vax-Before-Travel.com/MalariaOutbreasks.
Through the Ministry of Foreign Trade and Tourism (Mincetur), the Peruvian government recently presented a national "Safe Tourism" strategy to ensure a good travel experience for tourists visiting Peru.
The Safe Tourism program has three components: Security, Formalization, and Strengthening applied in Peru's 25 regions.
For example, Minister Luis Fernando Helguero announced on February 9, 2023, that critical tourist infrastructure works would be inaugurated in the San Martín region in the coming weeks for more than S/ 50 million (~$13 million).
Peru has also established safe tourist corridors from the airports to historic centers in cities like Cusco, Arequipa, Puno, and Tacna.
These actions are essential since protests continue across Southern Peru, including in Cusco, Arequipa, Puno, and Lima, as of mid-February 2023.
Unfortunately, Machu Picchu remains closed to visitors until further notice.
The U.K. says the following States of Emergency and curfews have been announced:
- A 60-day State of Emergency occurred on February 4, 2023, in Madre de Dios, Puno, Cusco, Apurimac, Arequipa, Moquegua, and Tacna regions.
- A 10-day curfew in the Puno region came into force from 8 pm to 4 am on February 4, 2023.
- A 30-day State of Emergency occurred on January 19, 2023, in Amazonas and La Libertad regions.
- A 30-day State of Emergency occurred on January 15 on the roads: the Carretera Panamericana Sur, the Carretera Panamericana Norte, the Carretera Central, the Corredor Vial Sur Apurimac-Cusco-Arequipa and the Corredor Vial Interoceanica Sur.
The U.K. confirmed on February 11, 2023, that travelers arriving in Peru should be aware that traveling to some parts of the country or returning to Lima may not be possible and should be prepared for delays or disruption.
And the U.S. Embassy in Peru website says visitors should avoid demonstrations, and should they encounter any, remain in a safe location. For emergencies involving American citizens in Peru, please email [email protected] or call +51-1-618-2000.

CBS News recently reported scientists in the U.S. are preparing to test the first poultry vaccine against bird flu (avian influenza).
Biden administration officials informed CBS News on February 9, 2023, they have now begun weighing an unprecedented shift in the U.S. strategy to counter the outbreak of bird flu since 2022.
The United States Department of Agriculture's Animal and Plant Health Inspection Service (APHIS) previously confirmed the Eurasian H5N1 strain appeared in North America in January 2022.
Since then, APHIS reported over 6,000 H5N1 detections in wild birds affecting 47 states and losing over 58.3 million birds as of February 8, 2023.
While the U.S. Food and Drug Administration has approved a bird flu vaccine for people, there is not one available for birds.
A spokesperson for Merck Animal Health indicated to CBS News the company has an "extensive, ongoing research program" developing vaccines that can work with Differentiating Infected from Vaccinated (DIVA) strategies.
This approach systematically searches for the virus among vaccinated flocks to prevent undetected spread among immunized birds.
The DIVA strategy uses an inactivated oil emulsion vaccine containing the same haemagglutinin (H) subtype as the challenge virus but a different neuraminidase (N).
The possibility of using the heterologous N subtype to differentiate between vaccinated and naturally infected birds was investigated by developing an "ad hoc" serological test to detect specific anti-N1 antibodies.
In addition, the FDA confirms the annual flu shot is not designed to protect people from avian influenza viruses.
Other bird flu vaccine news is posted at PrecisionVaccinations.
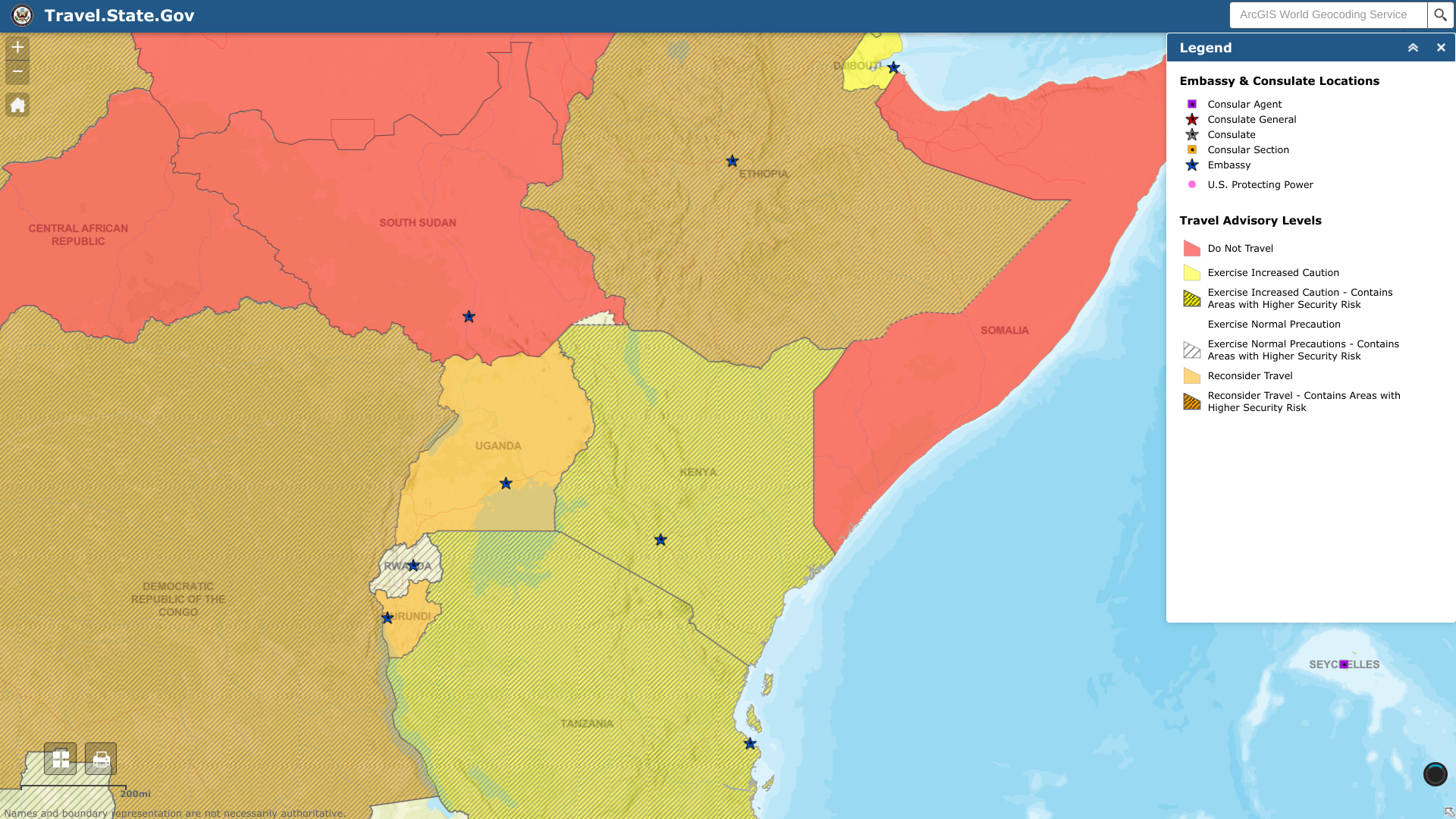
The U.S. Embassy Nairobi, Kenya, recently published a security alert for high-traffic areas frequented by foreigners and tourists.
On February 9, 2023, the Embassy wrote, Locations frequented by U.S. citizens and other foreigners and tourists in Nairobi and elsewhere in Kenya continue to be attractive targets for civil unrest.
And groups could create chaos at hotels, embassies, restaurants, malls and markets, schools, police stations, places of worship, and other places frequented by foreigners and tourists.
As a precaution, the government of the Republic of Kenya has increased its patrols.
Kenya is a prime destination for international travelers, welcoming over 800,000 guests in 2021.
Previously, the U.S. Department of State issued a Kenya - Level 2: Exercise Increased Caution advisory in December 2022.
This advisory suggests the following:
Do Not Travel to:
- Kenya-Somalia border counties and some coastal areas, and areas of Turkana County
Reconsider Travel to:
- Nairobi neighborhoods of Eastleigh and Kibera and specific areas of Laikipia County, and through Nyahururu, Laikipia West, and Laikipia North Sub-counties.
The State Department also suggests citizens in Kenya enroll in Smart Traveler Program to receive related alerts.
From a health perspective, the U.S. CDC issued advisories in 2022 for yellow fever and measles outbreaks in Kenya.
These are vaccine-preventable diseases available at travel clinics and pharmacies in the U.S.
Nykode Therapeutics ASA today announced a collaboration with the GOG Foundation, Inc. to conduct a clinical trial of VB10.16 in combination with an immune checkpoint inhibitor for treating advanced cervical cancer in the U.S.
VB10.16 is a potentially first-in-class off-the-shelf therapeutic cancer vaccine candidate in development for treating human papillomavirus type 16 (HPV16)-positive cancers.
The VB-C-04 trial will evaluate the combination therapies in patients with cervical cancer that have progressed following first-line treatment.
Nykode previously reported positive interim data from the Phase 2 VB-C-02 trial in Europe with women with heavily pre-treated advanced cervical cancer.
“Based on the positive interim data and in line with our potential registrational trial strategy, we look forward to starting the VB-C-04 trial in the U.S. The trial aims to provide a fast path to making VB10.16 available to patients,” stated Klaus Edvardsen, Chief Development Officer of Nykode Therapeutics, in a press release on February 10, 2023.
The cancer vaccine is designed based on Nykode’s Vaccibody™ technology platform of targeting antigens to antigen presenting cells.
VB10.16 has reported positive interim data from a Phase 2 trial in heavily pre-treated cervical cancer patients (NCT04405349).
The analysis demonstrated a favorable safety profile, with responses observed in both PD-L1 positive and negative patients (ORR 27% and 17%, respectively).
The vaccine-induced significant HPV16-specific T cell responses were associated with clinical responses.
The candidate has also demonstrated favorable clinical data in a Phase 1/2a study in pre-cancerous HPV16-induced high-grade cervical intraepithelial neoplasia (HSIL; CIN 2/3), demonstrating a statistically significant correlation of immune responses and clinical responses.
Since 1970, the GOG Foundation has conducted more than 350 clinical trials in the U.S. with 400 participating sites and 115,000 patients. The results of the GOG Foundation’s clinical trials have influenced the standard of care for numerous malignant gynecologic neoplasms.
Additional HPV vaccine news is posted at PrecisionVaccinations.com/HPV.

A clinical-stage biopharmaceutical company today announced the publication of a paper entitled "Single Dose of Recombinant Chimeric Horsepox Virus (TNX‐801) Vaccination Protects Macaques from Lethal Monkeypox Challenge" in the journal peer-review journal Viruses.
Tonix Pharmaceuticals Holding Corp. confirmed the publication demonstrates that a single dose vaccination with TNX‐801 was effective at protecting non-human primates from infection with the mpox virus.
David Evans, Ph.D., FCAHS, Professor and former Vice-Dean (Research) Faculty of Medicine and Dentistry at the University of Alberta and an investigator in the study and author of the publication, stated in a press release on February 9, 2023, "It is often forgotten that vaccines don't always produce sterilizing immunity and so it's very exciting to be able to report that a horsebox-based vaccine works so well in such a challenging infection model."
The publication describes data from animals in which eight of eight vaccinated with TNX-801 were fully protected with sterilizing immunity from a challenge with intra-tracheal monkeypox (central African, Congo Basin, clade).
These data show that the immunity generated by TNX‐801 was able to protect against a lethal challenge with the mpox virus and is the first study to demonstrate the efficacy of TNX‐801 vaccination against the mpox virus challenge in a non‐human primate model.
Synthetic horsepox virus is the basis for the Company's TNX-801 vaccine in development to protect against mpox and smallpox and for the Company's Recombinant Pox Virus platform to protect against other pathogens.
Additional mpox vaccine development news is posted at PrecisionVaccinations. And MpoxToday.com publishes updated research.

The Bayelsa State Government in Nigeria recently announced it would do everything within its power to ensure that Bayelsans have access to yellow fever (YF) vaccines.
Nigeria offers the Stamaril® live, attenuated YF vaccine.
The Tribue Online reported on February 3, 2023, Commissioner for Health Dr. Pabara Newton Igwele stated the YF disease was more frightening than COVID-19 as it does not have any special drugs for curative measures.
He further noted that YF is caused by a virus transmitted through bites of infected mosquitoes.
And a life-threatening disease, explaining that its symptoms manifest in bleeding from the mouth, eye, and nose, which, if not properly managed, could resolve to death.
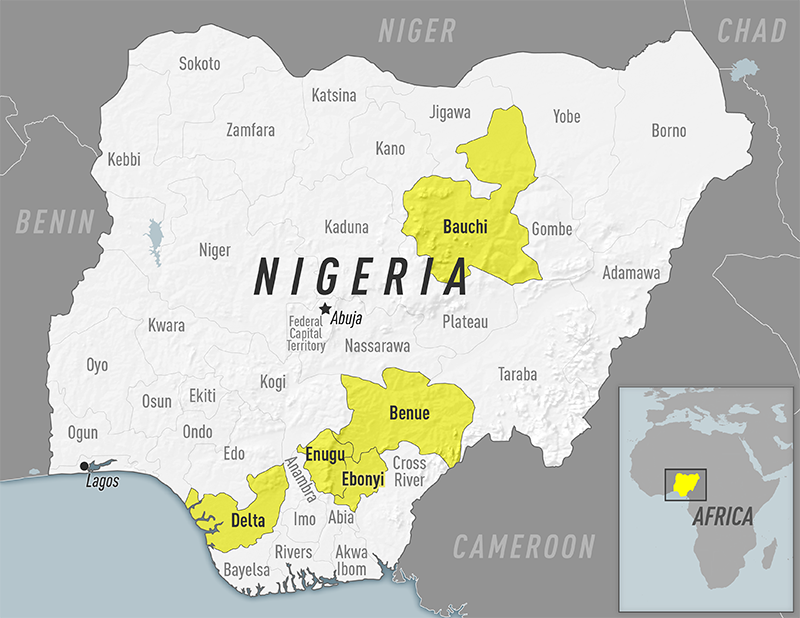
According to the U.S.Centers for Disease Control and Prevention (CDC), the Nigeria Centre for Disease Control has been reporting yellow fever outbreaks in multiple states (Bauchi, Benue, Delta, Ebonyi, and Enugu) since 2020.
Beginning in late January 2018, more than 25 million people were scheduled to be vaccinated in Nigeria.
As of 2023, the CDC says travelers to Nigeria should take steps to prevent yellow fever by getting vaccinated at least ten days before travel and taking steps to prevent mosquito bites.
The Stamaril vaccine is not offered in the U.S. However, certified clinics and pharmacies offer the YF-Vax® vaccine in the U.S.
Following vaccination, the yellow fever card (International Certificate of Vaccination or Prophylaxis) becomes certified.