Search API
Millions of people's health is at risk by the mosquito-transmitted, vaccine-preventable chikungunya virus (CHIKV), but most Americans are unaware of its global impact.
In-person information about a newly approved chikungunya virus (CHIKV) vaccine will be shared at the Walter E. Washington Convention Center in Washington, D.C., from April 1-4, 2024.
Valneva SE, a France-based specialty vaccine company, today announced it will present its single-shot chikungunya vaccine for adults, IXCHIQ®, and participate in a panel discussion on efforts to eradicate chikungunya outbreaks at the 24th World Vaccine Congress.
XCHIQ®, the world's first and only chikungunya vaccine approved in the U.S., was recently recommended by the CDC's Advisory Committee on Immunization Practices for international travelers to at-risk areas.
As of March 21, 2024, CHIKV was identified in nearly 115 countries, primarily in the Region of the Americas. As of December 2023, approximately 460,000 CHIKV cases and 360 related deaths have been reported worldwide.
On the evening of April 2, Valneva will attend the Vaccine Industry Excellence Awards ceremony, where the vaccine is a finalist for the Best Prophylactic Vaccine award for IXCHIQ®.
And on April 3, Valneva's Chief Medical Officer, Dr. Juan Carlos Jaramillo, will participate in the "Vaccine Development and Efforts towards Eradicating Chikungunya" panel discussion.
For further details, contact Laetitia Bachelot-Fontaine, VP, Global Communications and European Investor Relations, at [email protected]

Merck today announced plans to initiate clinical development of a new investigational multi-valent human papillomavirus (HPV) vaccine designed to provide broader protection against multiple HPV types.
Separately, the company also plans to conduct clinical trials to evaluate the efficacy and safety of a single-dose regimen of GARDASIL®9 compared to the approved three-dose regimen.
“Evidence continues to emerge showing the importance of GARDASIL and GARDASIL 9 to public health,” said Dr. Eliav Barr, senior vice president, head of global clinical development, and chief medical officer, Merck Research Laboratories, in a press release on March 13, 2024.
“These significant investments build upon our leadership and, importantly, provide the opportunity to further impact the global burden of certain HPV-related cancers and diseases.”
Merck announced in February 2024 that GARDASIL/GARDASIL 9 vaccine sales reached about $8.9 Billion;
The World Health Organization (WHO) recommends vaccinating against HPV to prevent infections and associated cancers.
As of March 2024, the WHO has listed six licensed HPV vaccines protect males and females against cancers caused by HPV. These bivalent, quadrivalent, and nonavalent HPV vaccines are available in 140 countries.
Furthermore, the WHO and the United Kingdom have endorsed the single-dose regimen.

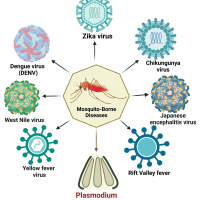
Scientists from Duke-NUS Medical School (Duke-NUS) have developed a new approach using the Zika virus to destroy brain cancer cells and inhibit tumor growth while sparing healthy cells.
Using Zika virus vaccine candidates developed at Duke-NUS, the team discovered how these strains target rapidly proliferating cells over mature cells—making them an ideal option to target fast-growing cancerous cells in the adult brain.
Zika virus is one such option in early development. The Duke-NUS team used Zika virus live-attenuated vaccine (ZIKV-LAV) strains, which are "weakened" viruses with limited ability to infect healthy cells but can still increase and spread within a tumor mass.
"We selected Zika virus because it naturally infects rapidly multiplying cells in the brain, allowing us to reach cancer cells that are traditionally difficult to target. Our ZIKV-LAV strains also replicate themselves in brain cancer cells, making this a living therapy that can spread and attack neighboring diseased cells," said Dr. Carla Bianca Luena Victorio, first author of the paper and Senior Research Fellow at the Cancer & Stem Cell Biology Programme at Duke-NUS, in a press release on March 8, 2024.
Their study's findings, published in the Journal of Translational Medicine in February 2024, potentially offer a new treatment alternative for brain cancer patients who currently have a poor prognosis.
Glioblastoma multiforme is the most common malignant brain cancer, with more than 300,000 patients diagnosed annually worldwide. Survival rates for such patients are poor (around 15 months), mainly due to the high incidence of tumor recurrence and limited treatment options.
For such patients, oncolytic virotherapy—or the use of engineered viruses to infect and kill cancer cells—may address the current therapeutic challenges.
Separately, several Zika vaccine candidates are conducting clinical research as of March 2024.
A preventive vaccine targeting Zika is in demand since 36,738 Zika cases were reported in 2023.
In the Americas, the highest proportion of Zika cases was reported in Brazil (35,041), followed by Bolivia, Belize, Columbia, Paraguay, and Venezuela.