Search API
Communities in India have voiced strong interest in accessing HIV self-testing, says the World Health Organization (WHO).
The WHO today announced it recommends HIV self-testing (HIVST) as an important approach to address gaps in HIV diagnoses, including among key populations in India.
HIVST can also generate demand for prevention services and facilitate pre-exposure prophylaxis delivery.
The first of the United Nations’ 95-95-95 targets to end the HIV epidemic is for 95% of people living with HIV to know their HIV status by 2025. HIV testing is therefore essential to achieving “the first 95”.
A report launched in New Delhi in 2022 showed HIVST is acceptable to key populations and their partners in India.
In the U.S., clinicians are recommended to screen for HIV infection in all pregnant women, including those who present in labor or at delivery and whose HIV status is unknown.
And screening is endorsed for certain adolescents and adults who are at increased risk of HIV infection.
Globally, 98 countries now have policies supportive of HIVST, and 52 are routinely implemented, yet many countries have not yet introduced HIVST as a routine approach.
Until HIV-preventive vaccine candidates are approved, HIVST is a key component to reducing infections.

Eisai Co., Ltd. today announced that the U.S. Veterans' Health Administration (VHA) is providing coverage of LEQEMBI™ to veterans living with early stages of Alzheimer's disease (AD).
As of March 13, 2023, VHA healthcare professionals meeting the criteria set forth by the VHA can prescribe LEQEMBI to veterans who fit the VHA's standards and the U.S. Food and Drug Administration's (FDA) current label.
The FDA-approved LEQEMBI under the accelerated approval pathway in January 2023, and was launched in the U.S. on January 18, 2023.
LEQEMBI is a humanized immunoglobulin gamma 1 monoclonal antibody directed against aggregated soluble (protofibrils*) and insoluble forms of amyloid beta (Aβ),
Treatment with LEQEMBI should only be initiated in patients with mild cognitive impairment or mild dementia stage of disease and confirmed presence of Aβ pathology, the population in which treatment was initiated in clinical trials.
After the first infusion, 38% of LEQEMBI-treated patients had transiently decreased lymphocyte counts to <0.9 x109/L compared to 2% on placebo, and 22% of LEQEMBI-treated patients had transiently increased neutrophil counts to >7.9 x109/L compared to 1% on placebo.
Furthermore, there are no safety or effectiveness data on initiating treatment at earlier or later stages of the disease than were studied.
Continued approval for this indication may be contingent upon verification of clinical benefit in a confirmatory trial.
LEQEMBI is not a vaccine but is therapeutically administered via infusion.
In the event of an infusion-related reaction, the infusion rate may be reduced, or the infusion may be discontinued, and appropriate therapy initiated as clinically indicated. Prophylactic treatment with antihistamines, acetaminophen, nonsteroidal anti-inflammatory drugs, or corticosteroids prior to future infusions may be considered.
The FDA has not approved an Alzheimer's disease vaccine as of March 2023.
Parents living in St. Thomas, Elgin, and Oxford counties in Ottawa were recently advised to be alert to respiratory symptoms, which are particularly dangerous in young children.
Symptoms of the vaccine-preventable disease pertussis start with a runny nose or nasal congestion, sneezing, mild cough, and mild fever.
Southwestern Public Health, which is located between Detroit and Toronto, announced on March 8, 2023, parents and guardians should keep themselves and their children up to date with the pertussis vaccine after a recent dramatic rise in cases in the region.
"Our region has seen 82 confirmed cases of pertussis between January 2022 and February 28, 2023. This represents about 40% of the provincial total from that time period."
"Combine this with the number of children who are unvaccinated or under-vaccinated, and I am concerned in particular for the youngest members of our community," says Dr. Ninh Tran, Medical Officer of Health for Southwestern Public Health, in a related press release.
Pertussis, commonly known as whooping cough, is a vaccine-preventable disease.
This vaccine is routinely administered to children along with protection from polio, tetanus, and diphtheria (DTaP).
In the U.S., DTaP vaccines such as Boostrix are offered at clinics and pharmacies.
Pertussis is very contagious and spreads via droplets from the noses and mouths of those who are infected.
The cough, which can last anywhere from 2 – 8 weeks, gets progressively worse and may lead to vomiting or trouble breathing and coughing up mucous. It can often be recognized by the loud "whooping" sound that occurs when the child is inhaling after a coughing spell.
Untreated pertussis in infants can lead to hospitalization, brain damage, and death.
Furthermore, new research indicates an expecting mother can take action to protect her future child.
According to an Original Investigation published by JAMA Pediatrics in February 2023, maternal Tdap vaccination reduces pertussis burden in infants (2 months).
"I have two asks of our local parents."
"The first is that you make yourself familiar with the symptoms of pertussis and seek medical care if your child has these symptoms."
"It can be treated with antibiotics, and after five days on the treatment, the person can no longer spread the disease to others."
"Second, please contact your family health care provider or Southwestern Public Health to get your child's routine vaccinations up to date."
"The vaccine is free, and we have openings in our clinics throughout the month of March," adds Tran.
Ottawa residents requiring a public health vaccination clinic appointment can book online at www.swpublichealth.ca/booking.

The U.K. National History Museum recently reported sea lions in Peru are among the latest victims of a version of the highly pathogenic avian influenza (HPAI) known as bird flu.
The HAPI virus has killed about 3,500 South American sea lions in Peru as of March 9, 2023.
The Peruvian government has reported that since November 2022, around 3% of the country's sea lions have died due to HPAI infections.
Peru, like many South American countries, believes HAPI was brought south by pelicans before jumping into the marine mammals.
In the Northern Hemisphere, Canada and the United States have reported multiple mammalian fatalities related to bird flu infections.
The United States Department of Agriculture and the World Animal Health Information System reported during March 2023, over 131 HAPI H5N1 detections of wild striped skunks, black bears, raccoons, and red foxes.
- The California Department of Fish and Wildlife received confirmation on February 15, 2023, that an adult bobcat died from the Eurasian strain of HPAI H5N1.
- The Colorado Parks and Wildlife confirmed on February 9, 2023, several cases of HPAI in free-ranging wildlife (black bear, skunk, mountain lion).
- The Montana Department of Fish, Wildlife, and Parks confirmed on January 17, 2023, three juvenile grizzly bears tested positive for HAPI.
While there are no vaccines that protect birds or mammals from H5N1 infections, there are bird flu vaccines for humans.
In the U.S., the Food and Drug Administration authorized CSL Seqirus' Audenz™ vaccine on January 31, 2020, and RAPIVAB® in 2022.
And the U.S. government has financially supported the development of newer bird flu vaccines for people.
Furthermore, the government reminds everyone that annual flu shots are effective against certain types of influenza, but they are not effective against bord flu viruses.

The Centers for Disease Control and Prevention (CDC) recently released a Morbidity and Mortality Weekly Report (MMWR) titled: Interim Clinical Treatment Considerations for Severe Manifestations of Mpox—United States, February 2023.
Published on March 3, 2023, this MMWR provides updated clinical treatment considerations about using therapeutic countermeasures to treat severe mpox cases.
Until data gaps are filled through randomized controlled studies and other carefully controlled research studies, this MMWR represents the best available information about human mpox treatment.
Previously, the CDC updated the U.S. National Mpox Vaccination Strategy on February 6, 2023.
The CDC continues to recommend people who have been exposed to the Mpox virus and people who may be more likely to contract mpox should be vaccinated.
The JYNNEOS® vaccine is available at certain clinics and pharmacies in the U.S.
Mpox is a disease caused by infection with the Monkeypox virus, an Orthopoxvirus in the same genus as the Variola virus, which causes smallpox.
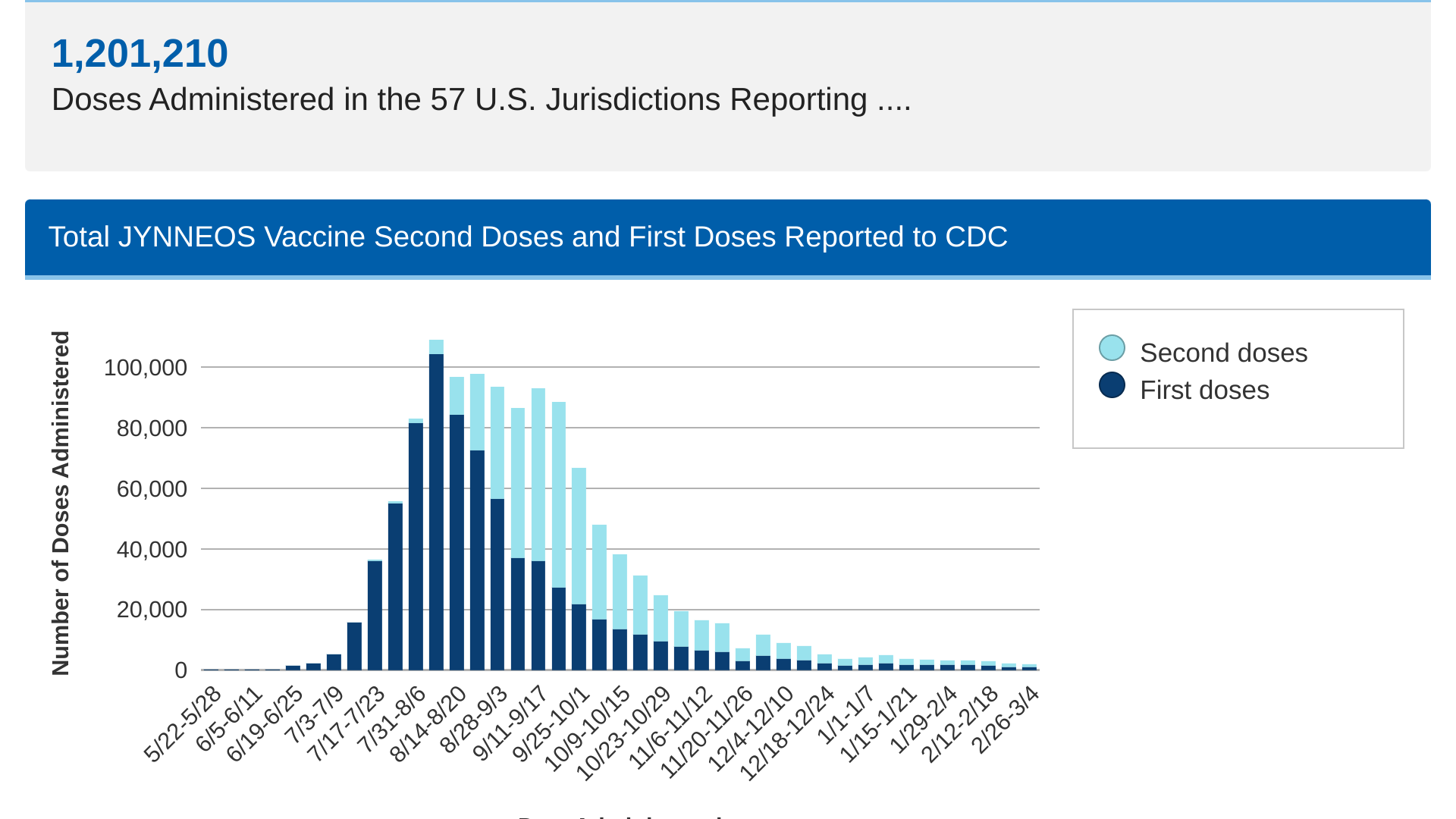
In 2022, a global outbreak involving mpox clade IIb was detected sourced from the Canary Islands in early May. Since then, 1,201,210 JYNNEOS doses have been administered in the 57 U.S. Jurisdictions reported data.
The New York State Department of Health today announced New Yorkers are planning to travel abroad to Israel and other countries with circulating poliovirus to get fully immunized against polio.
Recently, Israel's Ministry of Health confirmed four children had tested positive for poliovirus in Northern Israel.
Furthermore, Israel has reported widespread detection of poliovirus in wastewater systems.
This latest outbreak follows one that was detected in February 2022 when seven children tested positive for poliovirus in Jerusalem.
With the latest polio case, New York health officials have contacted their Israeli counterparts to ensure a coordinated response.
As of March 10, 2023, enhanced precautions are encouraged when visiting the United Kingdom, Ukraine, Afghanistan, Pakistan, Indonesia, Nigeria, Côte d'Ivoire, several other central African countries, and Israel.
In the U.S., polio vaccines are available at health clinics and community pharmacies.
Additional polio outbreak 2023 news is posted at Vax-Before-Travel.

U.S. Consulate General Amsterdam, Netherlands, announced significant demonstrations are expected to occur on March 11, 2023, and advised visitors to avoid these crowds.
Furthermore, U.S. government personnel in Amsterdam have also been advised to avoid the pending, conflicting demonstrations near The Hague in Zuiderpark and around Koekamp near Central Station, including on the A12.
To help bypass the crowds, the Consulate advises U.S. citizens to enroll in the Smart Traveler Enrollment Program to receive digital security updates related to these events.
Or visit the U.S. Consulate General Amsterdam at Museumplein 19, 1071 DJ Amsterdam.
And to call 1-1-2 for emergency service from Dutch Police, Rescue, and Fire Departments.
Recently the U.S. Department of State reissued its Level 2: Exercise Increased Caution alert on March 9, 2023, highlighting the ongoing civil unrest in The Netherlands.
And from a health perspective, the U.S. CDC suggests various travel vaccines for visiting the Netherlands.

Reuters recently confirmed GSK plc expects to launch its respiratory syncytial virus (RSV) vaccine in the U.S. in 2023 without supply constraints.
"We are ready to launch without capacity or supply constraints... to supply the market (from its plant in Wavre, Belgium) that we see," Phil Dormitzer, Global Head of Vaccines R&D at GSK, commented in an interview on March 8, 2023.
GSK plc previously announced that the U.S. Food and Drug Administration (FDA) vaccine voted that the available data support the safety and effectiveness of GSK's AREXVY™ vaccine candidate for preventing lower respiratory tract disease caused by RSV in adults aged 60 years and older.
The FDA Committee voted unanimously 12-0 on effectiveness and 10-2 on safety.
As of March 10, 2023, the FDA has yet to authorize AREXVY for use in the U.S.
Phil Dormitzer commented in a press release on March 1, 2023, "Today's vote brings us an important step closer to delivering one of the world's first vaccines for RSV, a respiratory virus that causes potentially debilitating disease and imposes a major burden on healthcare systems."
AREXVY is also under regulatory review by the European Medicines Agency, Japan's Ministry of Health, Labour and Welfare, and several other regulators, with initial decisions expected later in 2023.
RSV is one of the major remaining infectious diseases for which no vaccine or specific treatment is available for adults. Older adults are at high risk for severe disease due in part to age-related decline in immunity,
Additionally, RSV is a very serious disease in young children.
Unlike seniors, children already have FDA-approved RSV protection from a monoclonal antibody (Synagis®), with another version under final review.
The European Medicines Agency recommended marketing authorization for Beyfortus® (nirsevimab) in 2022 to prevent RSV lower respiratory tract disease in infants during their first RSV season when there is a risk of RSV infection in the local community.
Additional RSV vaccine candidates and antibody therapy news are posted at PrecisionVaccinations.com/RSV.