Search API
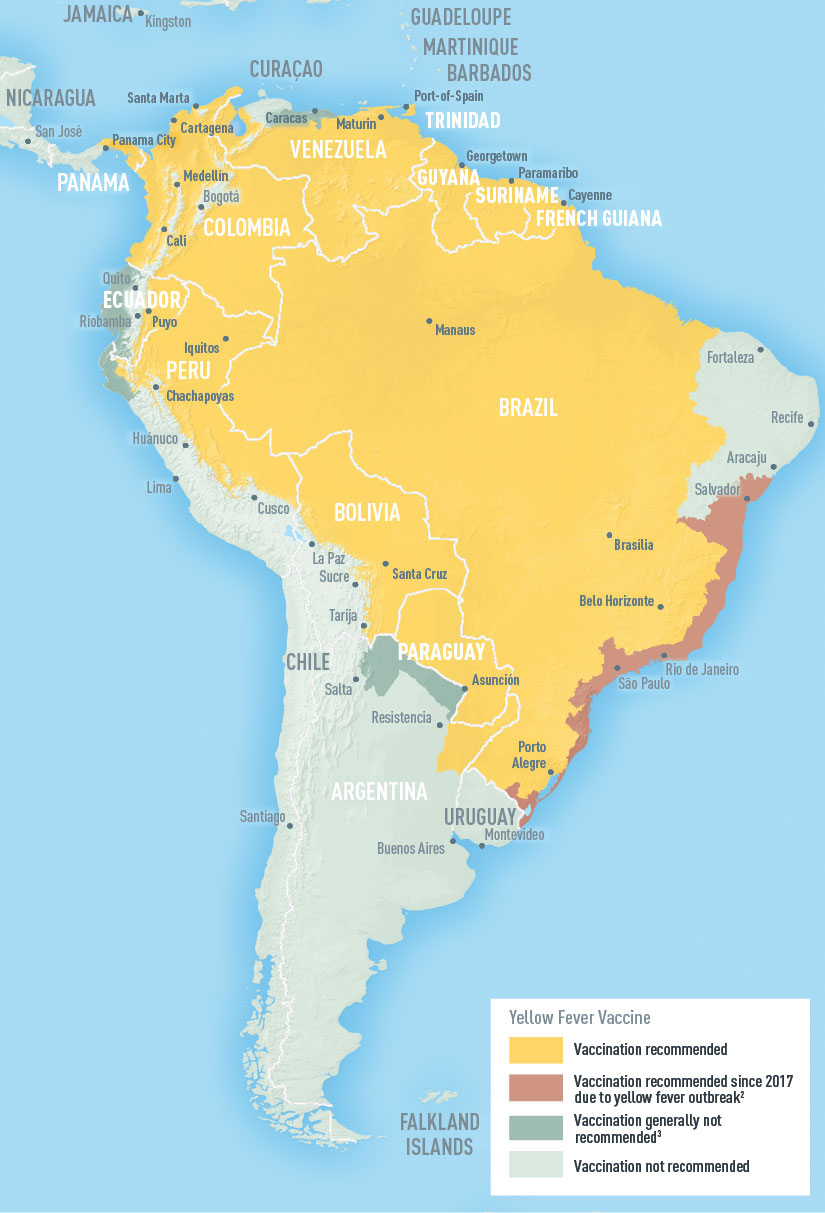
The yellow fever virus (YFV) is a reemerging global health threat in 2023, driven by several factors, including the increased spread of the mosquito vector.
Although protective YFV vaccines exist, recent outbreaks in South America indicate a void in treatment options in countries such as Brazil.
To establish innovative treatment options for patients with severe YFV infection, researchers recently tested 37 YFV-specific neutralizing monoclonal antibodies (mAbs) isolated from previously vaccinated humans.
They identified two capable of potently neutralizing multiple pathogenic primary YFV isolates.
Using hamster and nonhuman primate models of lethal YFV infection, they demonstrate that a single administration of either potently neutralizing mAbs during acute infection fully controlled viremia.
And it prevented severe disease and death in treated animals.
'Given the potential severity of YFV-induced disease, these results show that these antibodies could effectively save lives and fill a much-needed void in managing YFV cases during outbreaks, wrote these researchers in a Science Translational Medicines article published on March 29, 2023.
In the U.S., the YF-Vax® vaccine is available as of April 13, 2023, at certified clinics and travel pharmacies.
Furthermore, the International Certificate of Vaccination, known as the yellow card, is required to enter certain countries in 2023.
Various types of COVID-19 vaccines have been approved to reduce the disease burden during the recent pandemic. To better appreciate differences, South Korean researchers conducted an observational study to evaluate the effectiveness of Novavax's NVX-CoV2373 and BNT162b2 vaccines in protecting adults.
This non-peer-reviewed study was published on February 19, 2023, and compared the results from 3,019 recipients of NVX-CoV2373 and 3,027 recipients of BNT162b2 vaccines.
The 40-week risk ratios for recipients of the NVX-CoV2373 vaccine compared with recipients of the BNT162b2 vaccine were 1.169 (95% CI, 1.015 to 1.347) for laboratory-confirmed SARS-CoV-2 infection.
And 0.504 (95% CI, 0.126 to 2.014) for severe SARS-CoV-2 infection.
The estimated risk of severe infection was 0.001 events per 1000 persons (95% CI, 0 to 0.003) for the NVX-CoV2373 vaccine and 0.002 events per 1000 persons (95% CI, 0.001 to 0.006) for the BNT162b2 vaccine.
These researchers wrote this 'study identifies the reduced risk of SARS-CoV-2 infection and severe infection after receipt of three doses of either NVX-CoV2373 or BNT162b2 vaccines in Korean adults.
The study authors did not declare any competing industry interest.

The University of Oxford today announced the R21/Matrix-M™ malaria vaccine was licensed for use in Ghana by the country’s Food and Drugs Authority.
This announcement marks the first regulatory clearance for the R21/Matrix-M malaria vaccine for use in any country for children at the highest risk of death from malaria.
According to the press release on April 13, 2023, the R21/Matrix-M vaccine has demonstrated high levels of efficacy and safety in Phase II trials, including amongst children who received a booster dose of R21/Matrix-M at one year following a primary three-dose regime.
The R21/Matrix-M malaria vaccine is a low-dose vaccine that can be manufactured at scale and modest cost, enabling as many as hundreds of millions of doses to be supplied to African countries which are suffering a significant malaria burden.
According to the U.S. Centers for Disease Control and Prevention, malaria is a vaccine-preventable mosquito-borne disease caused by a parasite. Malaria vaccines like Mosquirix™ and R21 have been reported effective at preventing disease.
As of April 13, 2023, these malaria vaccines are unavailable in the U.S.
The U.S. government today announced it is releasing the National COVID-19 Preparedness Plan. This plan lays out the roadmap to help fight COVID-19 in the future.
'We look to a future when Americans no longer fear lockdowns, shutdowns, and our kids not going to school,' wrote the U.S. government on April 12, 2023.
'It's a future when the country relies on the powerful layers of protection we have built."
"And invests in the next generation of tools to stay ahead of this coronavirus."
The President's National COVID-19 Preparedness Plan focuses on four key goals, which are linked here.

INOVIO today announced that an abstract had been accepted for presentation for INO-4201 as an Ebola booster for Merck's Ervebo® (rVSV-ZEBOV) vaccine at the 33rd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID).
"We are pleased that lead investigator Dr. Angela Huttner will have the opportunity to share important new humoral and cellular response data at ECCMID from our recently completed Phase 1b trial of INO-4201 as an Ebola booster vaccine candidate for Ervebo," said Dr. Laurent Humeau, INOVIO's Chief Scientific Officer, in a press release on April 12, 2023.
INO-4201 is a DNA vaccine targeting Zaire Ebola virus (ZEBOV) glycoprotein, designed to prevent infection.
INO-4201 encodes for a synthetic consensus antigen encompassing ZEBOV genetic variability from various outbreak strains to broaden immune coverage for divergent ZEBOV variants.
The Ebola virus family includes four virus species that cause periodic outbreaks of a highly contagious and lethal human infectious disease called Ebola Virus Disease (EVD).
New research suggests dormant Ebola virus in a previously infected survivor could re-emerge up to nearly five years later and again allow human-to-human transmission.
The Ebola virus is classified as a Category A Priority Pathogen by the U.S. Centers for Disease Control and Prevention.
Also, the World Health Organization lists EVD as a priority for research and development in emergency contexts and coordinates planning to prevent and respond to Ebola epidemics.
Ebola vaccines have been approved and deployed in Africa.

The U.S. Centers for Disease Control and Prevention (CDC) today announced a previously unscheduled meeting of the Advisory Committee on Immunization Practices (ACIP) focused on COVID-19 vaccinations.
According to the CDC's website, on April 12, 2023, the ACIP will digitally meet on April 19, 2023, at 11 am EST. This meeting's agenda has yet to be posted for public review, and no registration is required to watch the webcasts.
A summary of recent changes (last updated March 16, 2023) is posted on this CDC page.
Updated on April 19, 2023, with the meeting's agenda.

Moderna Inc. recently confirmed it has five influenza vaccine candidates in clinical development. As of April 11, 2023, these flu shots include:
- mRNA-1010, a seasonal quadrivalent vaccine using strains recommended by the World Health Organization,
- mRNA-1011/1012, a seasonal penta-/hexa-valent vaccine candidate that includes more hemagglutinin antigens (e.g. H3, H1) to expand strain matching,
- mRNA-1020/1030, a seasonal vaccine candidate that includes neuraminidase antigens to target more conserved regions of the virus.
The Company's first vaccine candidate against influenza is mRNA-1010, developed in adults and is currently being evaluated in two Phase 3 trials.
The first Phase 3 trial (P301) was conducted in the Southern Hemisphere to evaluate the safety and non-inferior immunogenicity compared to a licensed flu vaccine.
The previously announced interim results from the P301 trial indicated that mRNA-1010 demonstrated superiority in geometric mean titers (GMT) for A/H3N2 and non-inferiority in GMT for A/H1N1.
However, mRNA-1010 did not meet non-inferiority for both influenza B/Victoria- and B/Yamagata-lineage strains.
But, mRNA-1010 demonstrated an acceptable safety and tolerability profile in the trial, and the independent Data and Safety Monitoring Board (DSMB) for P301 did not identify any safety concerns.
The second Phase 3 trial (P302) is being conducted in the Northern Hemisphere to evaluate the safety and non-inferior efficacy compared to a licensed flu vaccine.
The independent DSMB has completed the first interim analysis of efficacy and informed the Company that mRNA-1010 did not meet the statistical threshold necessary to declare early success and recommended that the trial continues with efficacy follow-up towards the next analysis.
The DSMB did not identify any safety concerns, and blinded follow-up for safety and efficacy is ongoing in this trial.
A preliminary immunogenicity analysis from a subset of participants in the P302 trial has also been completed.
In this analysis, mRNA-1010 demonstrated geometric mean titer ratios consistent with superiority against both influenza A strains (A/H1N1, A/H3N2) and consistent with non-inferiority against both influenza B strains (B/Victoria, B/Yamagata) relative to the licensed comparator.
The P302 study did not pre-specify success criteria for immunogenicity endpoints.
Additionally, the Company announced it had developed an update to mRNA-1010 that is expected to have improved immunogenicity against influenza B strains. It also announced plans to initiate a confirmatory Phase 3 trial in 2023.
Stéphane Bancel, Chief Executive Officer of Moderna, commented in a related press release, "With mRNA-1010, our first investigational vaccine against seasonal flu, we are encouraged by the consistently strong immunogenicity results against influenza A, and titers consistent with non-inferiority against influenza B strains in the most recent Phase 3 trial."
"With our mRNA platform and technology, as well as our agile manufacturing capabilities, we are confident that we can quickly develop safe and effective vaccines to address critical unmet needs."