Search API
ANI News posted a video that revealed Serum Institute of India's (SII) CEO Adar Poonawalla informed the local media that the institute recently produced 5-6 million doses of the protein-based CovoVax™ COVID-19 vaccine.
However, as of April 22, 2023, the demand for CovoVax vaccines is zero in Indian hospitals.
Poonawalla added, "Current (SARS-CoV-2) variants are mild and not severe... However, senior citizens can take booster doses as a precaution."
These vaccines may be needed as the WHO South-East Asia Region, which includes India, recently reported a (+654%) increase in COVID-19 cases and fatalities.
Maryland-based Novavax, Inc. and SII confirmed CovoVax vaccine was authorized in Indonesia on December 1, 2021, and in India on December 28, 2021.
In the U.S., the U.S. FDA confirmed on August 19, 2022, that the Novavax COVID-19 Vaccine was available to prevent COVID-19 in individuals 12 years of age and older.
Novavax COVID-19 vaccine brands are available in over 40 countries as of April 2023.

GlaxoSmithKline (GSK) Pharmaceuticals Ltd today announced the launch of its Shingrix® vaccine to prevent shingles (herpes zoster) and post-herpetic neuralgia in adults aged 50 years and above in India.
PTI reported Shingrix can provide at least ten years of protection against shingles, which is caused by the reactivation of the varicella-zoster virus, the same virus that causes chickenpox, GSK said in a statement on April 24, 2023.
In the U.S., the CDC's Morbidity and Mortality Weekly Report confirmed that on January 21, 2022, the Advisory Committee on Immunization Practices recommended two doses (0.5 mL each) to prevent herpes zoster and related complications in immunodeficient or immunosuppressed adults.
Singrix was initially approved in the U.S. in 2017 and Europe in 2018 and is now available in over 20 countries in 2023.

Health Canada today announced it had issued a Notice of Compliance approving BEYFORTUS™ (nirsevimab) for the prevention of respiratory syncytial virus (RSV) lower respiratory tract disease (LRTD) in newborns and infants during their first RSV season.
And children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
BEYFORTUS is not a vaccine.
It is an immunizing agent and a long-acting monoclonal antibody (mAB).
The approval on April 24, 2023, was based upon a BEYFORTUS™ clinical development program spanning three pivotal late-stage clinical trials.
Jason Lee, Head of Vaccines Medical Affairs, Sanofi Canada, commented in a press release, "Today is a historical day for RSV prevention as decades of research and development culminate in Canada's approval of the first immunization against RSV disease."
"BEYFORTUS, designed using a long-acting mAB, will help meet a vast unmet need in RSV prevention, providing parents with an option to protect their infants during the first RSV season."
Sanofi stated it is committed to making BEYFORTUS available to newborns and infants for the upcoming 2023/2024 RSV season.
In the U.S., government agencies are reviewing this mAB for potential authorization this year.
The U.S. Food and Drug Administration (FDA) approved an injectable mAB therapy (Synagis®) in 1998.
RSV is a common and highly contagious seasonal respiratory virus infecting almost all children. It is a leading cause of infant hospitalizations (79%), including infants born at term with no underlying health conditions.
RSV also burdens the health system, with most hospitalized infants needing supplemental oxygen and some requiring ICU admission.
RSV vaccine candidates remain under review in the U.S. and have not been U.S. FDA-approved.

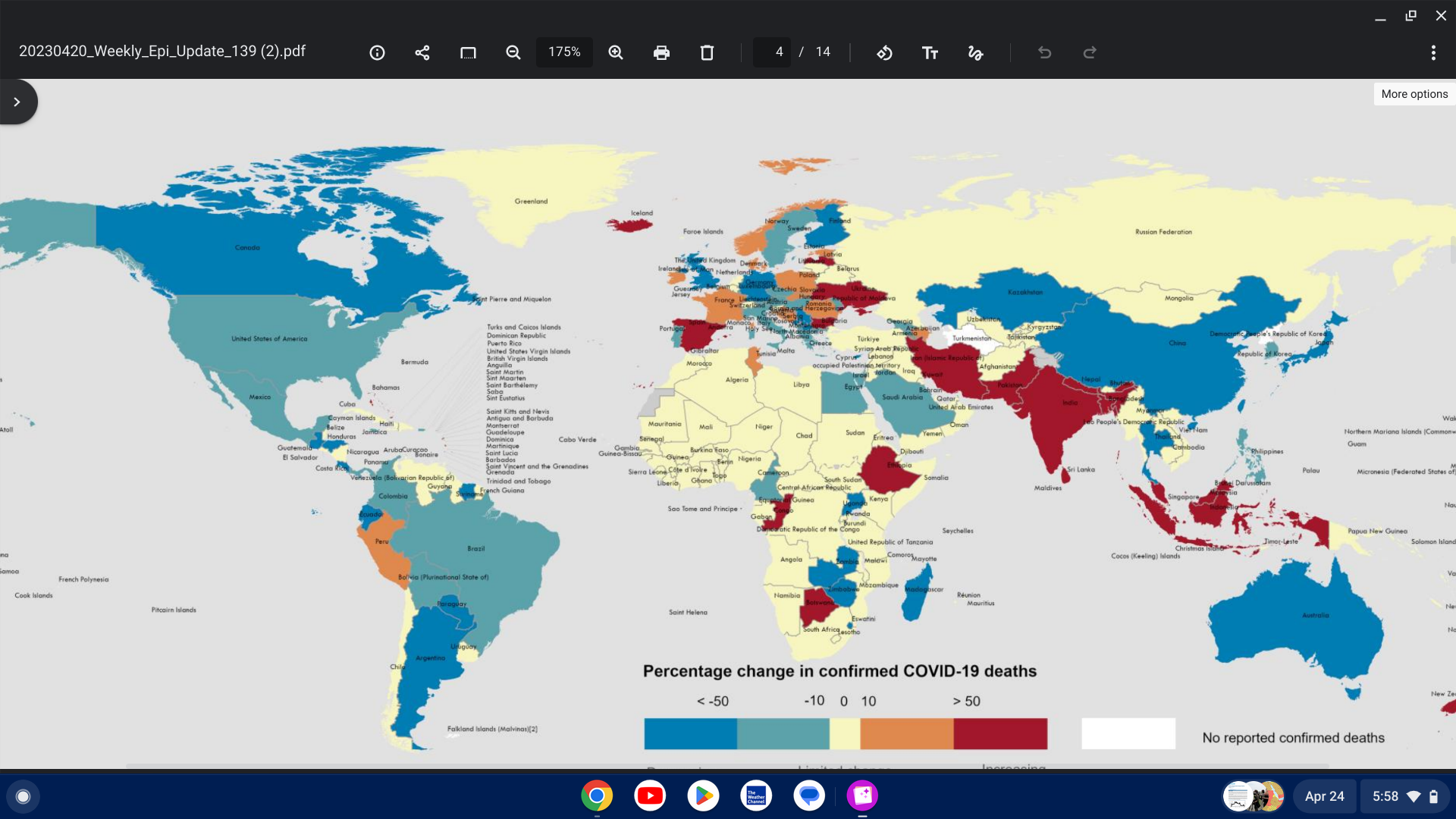
The World Health Organization (WHO) recently published update Edition #139 on the COVID-19 pandemic.
As of April 20, 2023, the WHO confirmed new COVID-19 cases and deaths decreased by 27% and 32%, respectively, compared to the previous month.
Contrary to the overall global trend, significant increases were seen in some WHO regions and countries, such as the South-East Asia Region (+654%), which includes India.
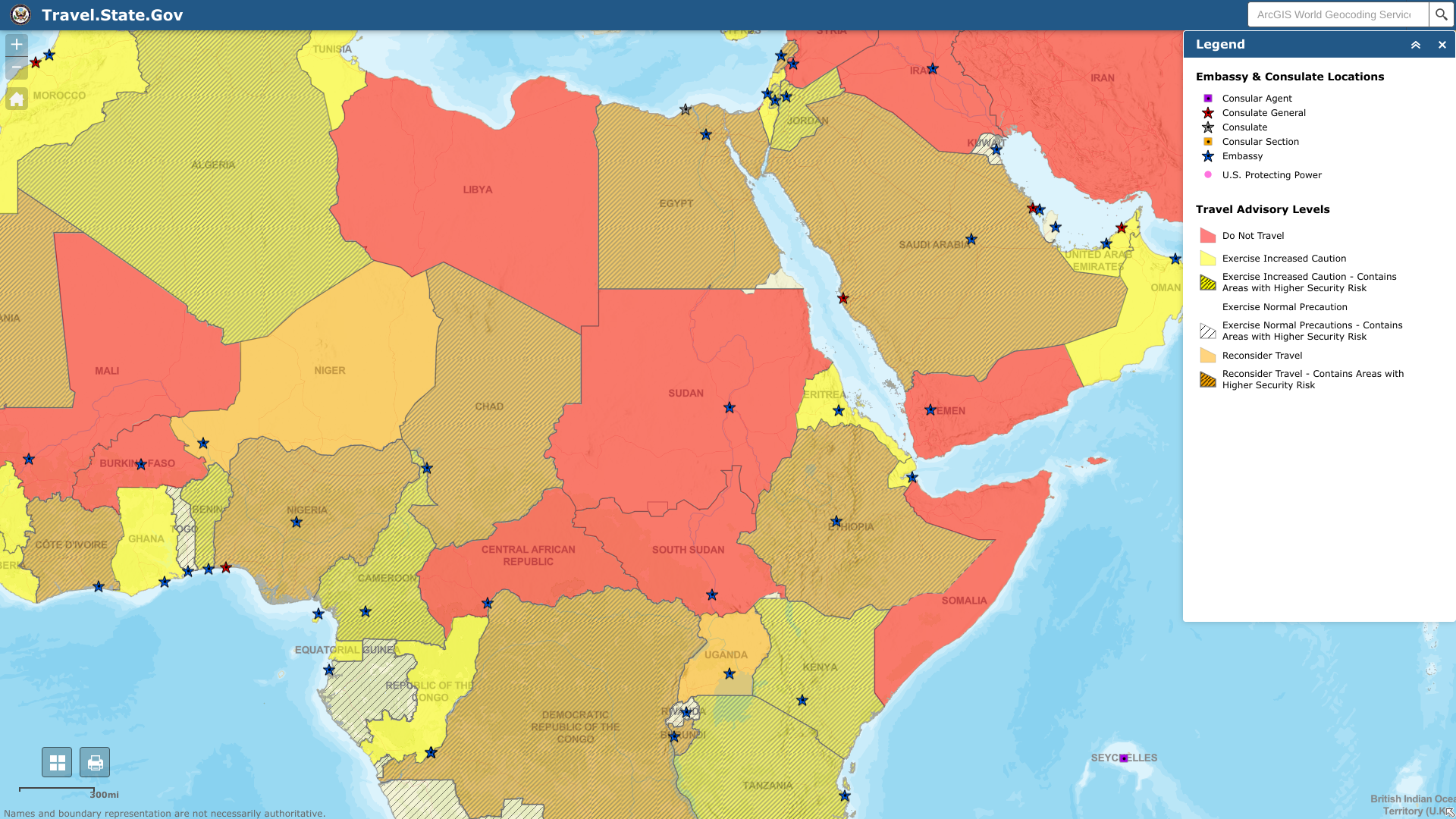
The U.S. Department of State issued a Level 4: Do Not Travel advisory for the Republic of Sudan in northeast Africa.
Announced on April 22, 2023, the travel alert confirmed the U.S. Embassy in Khartoum suspended its operations.
In addition, the State Department ordered the departure of U.S. direct-hire employees and eligible family members from the Embassy in Khartoum due to the continued civil unrest.
Specifically, the U.S. government cannot provide routine or emergency consular services to U.S. citizens in Sudan due to the current security situation.
We are aware of convoys departing Khartoum traveling towards Port Sudan. However, the Embassy cannot assist convoys, and traveling in any convoy is at your own risk, wrote the Embassy.
And if you travel to Sudan, exercise extreme care in all parts of the country, including Khartoum. Unfortunately, Khartoum's airport was closed due to the uncertain security situation.
Additionally, visitors are encouraged to enroll in the Smart Traveler Program to receive Alerts during an emergency.
From a health perspective, the U.S. CDC issued various travel alerts for Sudan's measles, polio, and dengue outbreaks.
Travel vaccination services are offered in Texas at local clinics and community pharmacies.
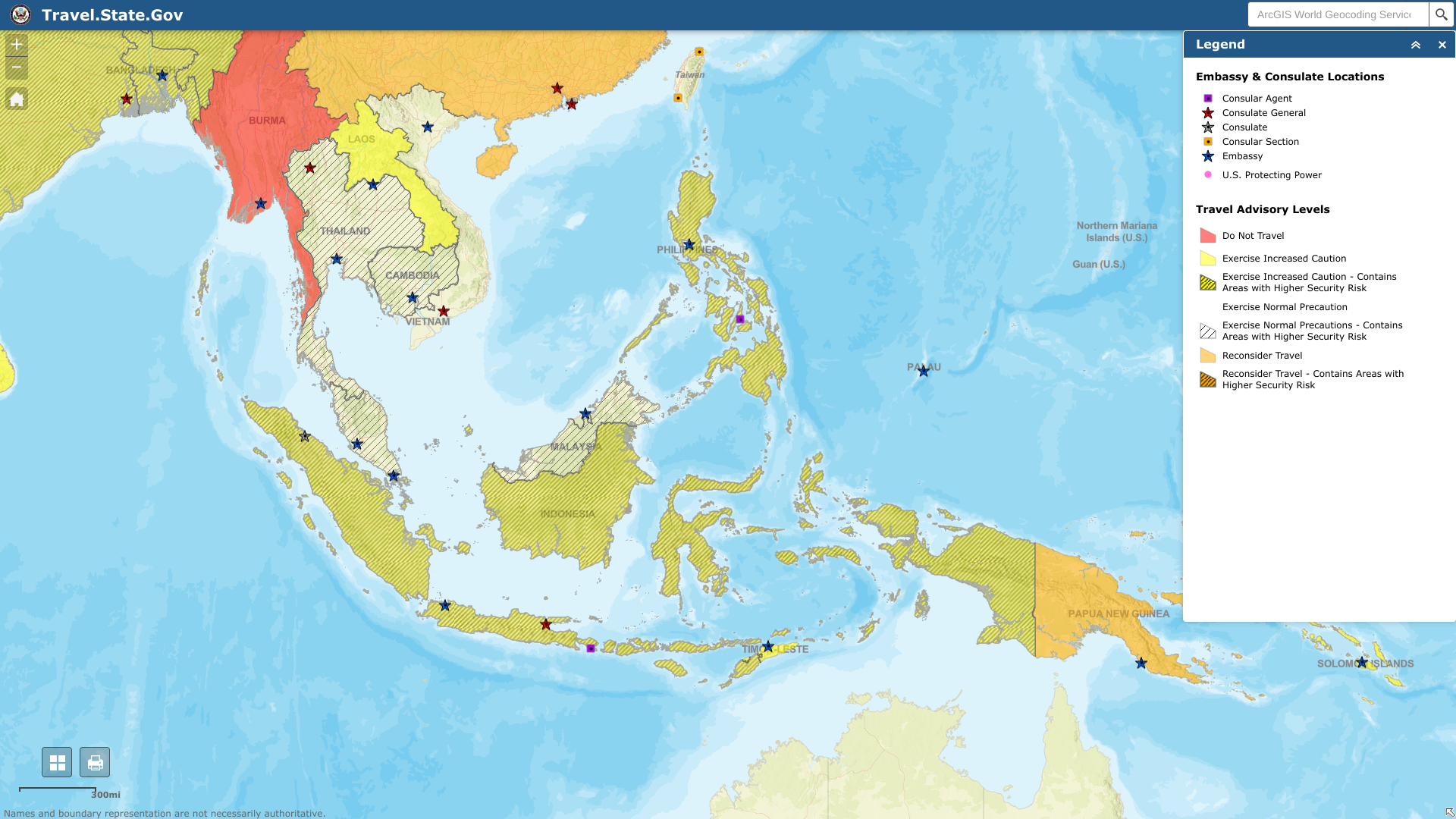
The World Health Organization (WHO) recently reported that the Republic of Indonesia's polio outbreak risk was assessed as high at the national level.
As of April 17, 2023, the WHO says the current polio outbreak in Indonesia poses a moderate regional risk and a low risk globally.
These assessments are related to the Indonesia Ministry of Health notifying the WHO of the detections of a circulating vaccine-derived poliovirus type 2 (cVDPV2) and acute flaccid paralysis (AFP) from the Purwakarta district in West Java province.
A total of four confirmed cases of VDPV2 have been reported in Indonesia since November 2022.
This includes three cases of cVDPV2 with AFP in Aceh province and one in West Java province.
Additionally, four healthy children in Aceh province were confirmed to have cVDPV2 on November 25, 2022.
Despite Indonesia having a strong capacity to respond to poliovirus outbreaks, there is a high susceptibility of the population to poliovirus type 2 after switching from trivalent to bivalent OPV.
WHO's International Travel and Health recommends that all travelers to polio-affected areas be fully vaccinated against polio.
And the WHO does not recommend any travel and/or trade restrictions to Indonesia based on the current information available for this event.
Indonesia has been included in recent U.S. CDC travel alerts for measles and polio.
In the U.S., polio vaccines are offered at health clinics and pharmacies in Texas.
The U.S. CDC's Weekly U.S. Influenza Surveillance Report published today highlighted negative news regarding influenza-associated pediatric mortality.
During Week #15, two influenza-associated pediatric deaths that occurred during the 2022-2023 season were reported this week.
This data increases the total number of pediatric flu deaths reported during the 2022-2023 flu season to 143.
Throughout the COVID-19 pandemic, there were very few pediatric deaths reported.
Pre-pandemic, during the 2019-2020 flu season, 199 deaths were confirmed by the CDC.
The positive news posted by the CDC on April 21, 2023, is the majority of influenza viruses tested are in the same genetic subclade as and antigenically similar to the influenza viruses included in this flu season's influenza vaccines.
The CDC continues to recommend that everyone ages six months and older get an annual flu vaccine as long as flu activity continues in the U.S.
As of March 4, 2023, the CDC reported that about 173 million flu shots had been distributed this flu season, about the same as 2021-2022.
And flu shots are advised for those traveling to countries where influenza cases have been reported, such as Brazil, Belize, Guatemala, Peru, and Europe.