Search API
The American Samoa Department of Health's latest Situation Report confirmed additional measles cases. As of April 30, 2023, the health department reported two confirmed measles cases and 52 probable.
American Samoa declared a public health emergency on April 24, 2023.
However, the U.S. CDC has not issued a related measles alert as of May 1, 2023. The CDC did issued a Global Measles Alert on April 6, 2023.
Measles is among the most contagious viral diseases known. Infected people are usually infectious from 4 days before until four days after rash onset. Therefore, the CDC suggests infants and unvaccinated adults traveling internationally should get one dose of a measles vaccine before traveling.
American Samoa is an unincorporated territory of the U.S. located in the South Pacific Ocean.
According to a non-peer-reviewed study, a strain of avian influenza H5N1 (bird flu) has been found to transmit between ferrets effectively.
Published by Biological Sciences on April 21, 2023, these researchers determined that uncharacterized genetic signatures may be important determinants of mammalian adaptation, and these Highly Pathogenic Avian Influenzas (HAPI) pathogenicity clade 2.3.4.4 viruses.
Clade 2.3.4.4b H5N1 viruses were first isolated from poultry and wild birds in Canada in December 2021. Since then, millions of birds have died worldwide.
And various mammals, such as bears, cats, dogs, seals, and others, have died from HAPI infections.
This study encouraged 'ongoing surveillance of circulating HPAI A(H5N1) viruses across species, including humans, should be a top priority to promptly identify viruses that may have pandemic or outbreak potential in mammals.'
While few human cases of infection with clade 2.3.4.4b viruses were reported as of April 30, 2023, the potential for spillover, particularly of viruses harboring mammalian adaptation signatures, remains a critical concern.
The U.S. CDC says the annual flu shot is not designed to protect people from bird flu viruses.
However, the U.S. government has already approved one vaccine (Audenz™) for this type of bird flu.

Blue Water Vaccines (VWV) Inc. recently announced the signing of a Sponsored Research Agreement with The University of Texas Health Science Center at San Antonio to fund a non-human primate ("NHP") study to evaluate the efficacy of BWV-401, a live attenuated, orally delivered Chlamydia vaccine candidate.
In this new effort, BWV will fund an NHP study to evaluate the efficacy of BWV-401 further and provide additional support for development towards human clinical trials.
In this upcoming study, NHPs will be vaccinated with BWV-401 and subsequently challenged against Chlamydia to validate the hypothesis that, along with being safe, this vaccine, when delivered orally, can elicit an effective immune response in the genital tract and can protect against Chlamydia infection.
BWV-401 utilizes a modified strain of Chlamydia to colonize in the gastrointestinal tract and has produced transmucosal protection against genital tract Chlamydia infection in mouse models without altering the gut microbiota.
"We are thrilled to initiate this study with our partners at UT Health Science Center San Antonio for BWV-401," said Joseph Hernandez, Chairman and Chief Executive Officer of BWV, in a press release on April 12, 2023.
"There remains a high unmet need for an efficacious Chlamydia vaccine to prevent the millions of infections worldwide each year."
According to the U.S. CDC, Chlamydia is the most frequently reported bacterial sexually transmitted infection in the U.S., with about 1.6 million new cases reported in 2020.
As of April 30, 2023, no U.S. FDA-approved Chlamydia vaccines.

The SCMP reported today that the Hong Kong Special Administrative Region of the People's Republic of China (HKSAR) recorded its fifth Mpox case. This 59-year-old patient had traveled to Guangdong province multiple times and was not linked to other Hong Kong cases as of April 30, 2023.
The initial Mpox patient was confirmed in September 2022 and presented with an infectious mononucleosis-like syndrome.
Since May 2022, over 100 countries have reported Mpox cases.
HKSAR's Health Department identified most of these cases among men. Mpox is caused by a sexually transmissible virus, and members of the public should seek medical attention if they experience symptoms such as fever, severe headache, muscle pain, swollen lymph node, mouth lesion, and rash.
Hong Kong's Mpox Vaccination Programme (JYNNEOS® (MVA-BN)) for high-risk groups began on October 5, 2022.
As of April 30, 2023, Mpox vaccines and treatments (TPOXX® (Tecovirimat)) were available in most countries.
However, recent research indicates breakthrough cases are being reported post-therapy.
Separately, the U.S. CDC recommends various routine and travel vaccination before visiting Hong Kong in 2023.

According to new data from the U.S. Transportation Security Administration (TSA), getting through airport security can be measured in minutes.
During March 2023, about 89% of TSA PreCheck® passengers waited less than 5 minutes to be processed at 200 airports in the U.S.
And the TSA makes this special service a family affair.
Children 12 and under can join a parent/guardian with TSA PreCheck® in the dedicated lanes.
Speeding through security is essential as the number of air travelers has returned to pre-pandemic levels.
As of the week ending April 27, 2023, TSA screening activity has matched 2019.
Passenger screening at the airport is part of TSA’s layered approach to security to get you safely to your destination. Search at this webpage to learn when TSA PreCheck® lanes are available at your airport.
Note: You do not need to get TSA PreCheck® if you already have Global Entry, NEXUS, SENTRI, or hold an active TWIC® or Commercial Driver’s License with an HME and you meet the TSA PreCheck® eligibility requirements.
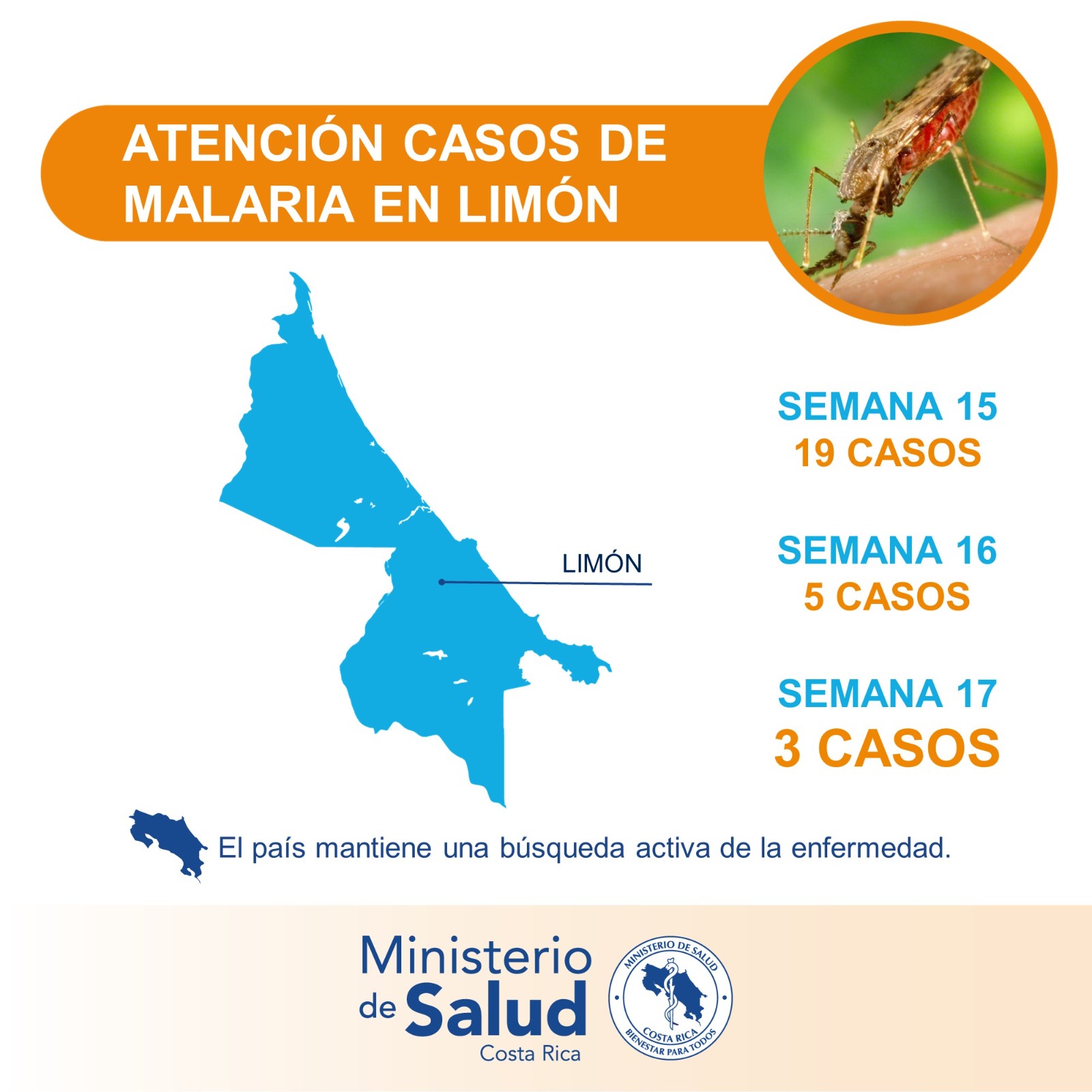
According to data recently announced from the Health Surveillance Directorate of the Costa Rican Ministry of Health, during epidemiological week #16, the Huetar Caribbean Region registered a marked decrease in malaria cases in the cantons of Limón and Pococí.
As of April 28, 2023, Limón reported 19 cases during week #15, while for week #16, 5 patients were registered, and for week #17, just 3 cases were reported, offering evidence of a significant decrease.
Furthermore, no malaria cases were identified in the canton of Talamanca, which corresponds to the South Caribbean, despite active inter-institutional searches in the border area with Panama and passive pursuits.
During the last weeks, the containment actions established by the Ministry of Health in conjunction with the Costa Rican Social Security Fund have been maintained, such as exhaustive tracking of all people in contact with confirmed cases.
Malaria is a disease caused by a parasite that spreads to humans through the bite of an infected mosquito.
Previously, the U.S. CDC issued an Alert - Level 2, Practice Enhanced Precautions confirming an outbreak of Plasmodium falciparum malaria in Limón and Alajuela Province.
Malaria can be prevented by taking a prescription antimalarial drug to kill the parasites, and in some countries, other than the U.S., malaria vaccines are available as of April 30, 2023.
The U.S. Centers for Disease Control and Prevention (CDC) today announced two additional influenza-associated pediatric fatalities were reported last week.
A total of 145 pediatric flu fatalities have been reported during the 2022-2023 season as of April 28, 2023.
The previous peak in pediatric fatalities was 199 during the 2019-2022 flu season before the COVID-19 pandemic closed society.
And based on National Center for Health Statistics Mortality Surveillance data available on April 27, 2023, about 7.5% of the fatalities during the week ending April 22, 2023 (week #16) were due to pneumonia, influenza, and/or COVID-19.
Most influenza viruses tested are in the same genetic subclade and antigenically similar to the influenza viruses included in this season’s influenza vaccine.
These flu shots remain available at most community pharmacies in the U.S.

The U.S. Food and Drug Administration (FDA) issued a letter today updating Pfizer Inc.'s COVID-18 vaccine authorization.
Addressed to Pfizer's Leslie Sands on April 28, 203, the new authorization for the Pfizer-BioNTech COVID-19 Vaccine, Bivalent for individuals six months through 4 years of age with certain types of immunocompromised, who have previously received three 0.2 mL doses (Pfizer-BioNTech COVID-19 Vaccine or Pfizer-BioNTech COVID-19 Vaccine, Bivalent):
- a fourth dose administered at least one month following the most recent dose;
- additional doses may be administered at the healthcare provider's discretion, considering the individual's clinical circumstances.
This issue was discussed during the U.S. CDC's Advisory Committee on Immunization Practices meeting on April 19, 2023.


