Search API
A clinical trial of an experimental universal influenza vaccine candidate developed by researchers at the National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center (VRC) has begun enrolling volunteers.
This Phase 1 trial will test the experimental vaccine, known as H1ssF-3928 mRNA-LNP, for safety and its ability to induce an immune response.
“A universal influenza vaccine would be a major public health achievement and could eliminate the need for both annual development of seasonal influenza vaccines, as well as the need for patients to get a flu shot each year,” said Acting NIAID Director Hugh Auchincloss, M.D., in a press release on May 15, 2023.
“Moreover, some strains of the influenza virus have significant pandemic potential. A universal flu vaccine could serve as an important line of defense against the spread of a future flu pandemic.”
A similar vaccine developed by researchers at NIAID’s VRC has already shown positive results in early clinical trials.
Both vaccines use a specific portion of a flu protein, hemagglutinin (HA), to induce a broad immune response against influenza.
While one portion of the HA protein, known as the head, tends to change as the flu virus spreads and evolves, a more stable portion, known as the stem, evolves very slowly and is very similar across many different types of the flu virus.
Researchers hope to induce long-term immunity against a broad range of flu viruses by using the HA stem as the basis for a vaccine.
Unlike the VRC’s earlier vaccine, the H1ssF-3928 mRNA-LNP vaccine candidate uses an mRNA platform.
By developing and testing various platforms for a universal flu vaccine, researchers are more likely to find one that is safe and provides strong and broad immunity against various strains.
Additional flu shot and influenza vaccine development news is posted at Precision Vaccinations.

YS Biopharma Co., Ltd. today announced its PIKA Rabies Vaccine candidate received Phase 3 clinical trial approval from the Drug Regulatory Authority of Pakistan.
The PIKA Rabies Vaccine has the potential to become the first accelerated three-visit one-week regimen, superior to the currently available vaccine with a five-visit one-month or three-visit three-week regimen.
Dr. Zenaida Mojares, Chief Medical Officer of YS Biopharma, commented in a press release on May 16, 2023, "Our progress enables us to advance towards our mission of providing innovative and efficacious vaccines in the fight against a vaccine-preventable rabies disease, with almost 100% case fatality rate."
Rabies is a zoonotic infection that is a vaccine-preventable viral disease. Unfortunately, almost 60,000 people worldwide die from rabies each year.
The number of human rabies deaths in the United States has been steadily declining since the 1970s thanks to animal control (bats and dogs) and vaccination programs, says the U.S. CDC.
Up to 95% of human deaths occur in Africa and Asia, where dog rabies is poorly controlled, says the WHO.
The Company has completed Phase 1 and 2 clinical trials of its PIKA Rabies Vaccine in Singapore. Another Phase 1 trial was also conducted in China. All three trials have shown that the PIKA rabies vaccine is safe, tolerable, and immunogenic.
In the U.S., various rabies vaccines are available at clinics and community pharmacies.
Note: Starting February 1, 2023, the temporary suspension for dogs entering the U.S. from high-risk countries for dog rabies was extended. This includes dogs arriving from countries without a high risk of rabies if the dogs have been in a high-risk country in the past six months.
Emergent BioSolutions today announced it had completed the sale of its travel health business to Bavarian Nordic and may receive up to $380 million in potential future payments.
Bavarian Nordic acquired the rights to Vivotif®, the licensed typhoid vaccine, and Vaxchora®, the licensed cholera vaccine, and the development-stage chikungunya vaccine candidate CHIKV VLP.
These travel-related vaccines are part of an estimated international market growth rate (9.9%) thru 2028. This data indicates millions of travelers are under-vaccinated before visiting disease-endemic countries.
Bavarian Nordic also acquired manufacturing facilities in Bern, Switzerland, and development facilities in San Diego, California.
"This deal achieves two significant outcomes key to our mission and future success," said Robert G. Kramer, Emergent president, and chief executive officer, in a press release on May 15, 2023.
Other market research reports indicate an uplift in international travel is coming.
Such as the Expedia Group's Traveler Insights reveals traveler searches increasing globally by 25% in Q1 2023, which means travelers are looking toward mid-year getaways.

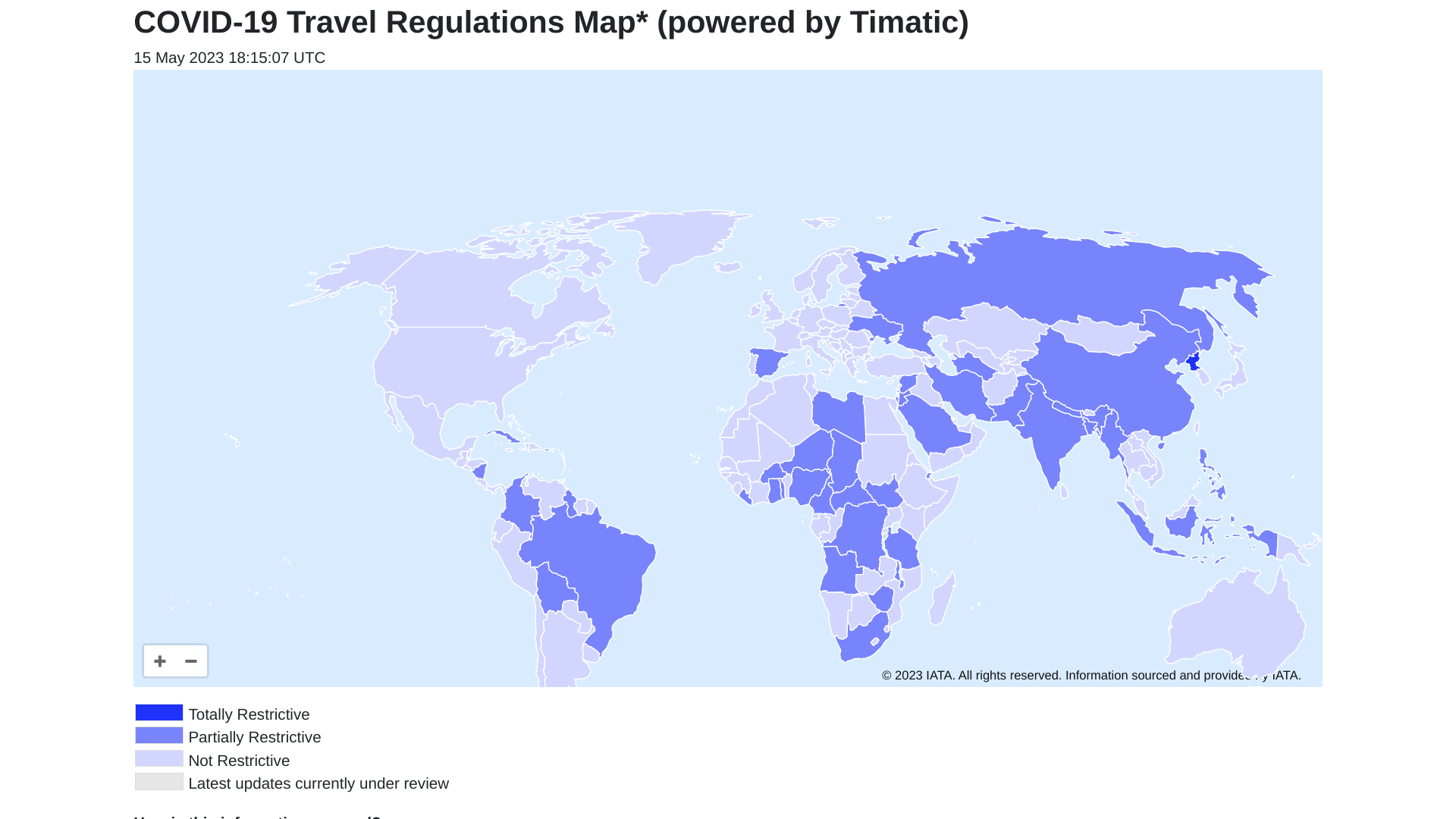
The Timatic interactive world map is designed to guide you through identifying requirements and is made available for information purposes only as of May 15, 2023.
This COVID-19 map is updated regularly, and due to the ever-changing nature of the regulations, we strongly advise that you check with your airline before you travel.
Recently, the World Health Organization (WHO published its weekly epidemiological update #142, which confirmed over 2.7 million new cases and over 17 000 deaths were reported in the last 28 days.
This data indicates a decrease of 14% and 17%, respectively, compared to the previous period.
The WHO says the global picture is mixed at the regional level, with increases in reported cases seen in the South-East Asia and Western Pacific regions and decreases in other areas.
As of May 7, 2023, over 765 million confirmed cases and over 6.9 million deaths have been reported globally.
Furthermore, various COVID-19 vaccines remain available in most countries.
The U.S. Centers for Disease Control and Prevention (CDC) announced a Health Alert Network Health Update today to inform clinicians and public health agencies that the mpox outbreak is not over.
As of May 15, 2023, the CDC continues to receive reports of mpox cases that reflect ongoing community transmission in the U.S. and Europe (France).
For example, from April 17 to May 5, 2023, a total of 12 confirmed and one probable mpox case was reported to the Chicago Department of Public Health.
Unfortunately, nine (69%) of 13 cases were among men who had received both doses of the U.S. FDA-approved JYNNEOS® (MVA-BN) vaccine.
The travel history was available for nine men; 4 recently traveled (New York City, New Orleans, and Mexico).
Although vaccine-induced immunity is not complete, vaccination continues to be one of the most important prevention measures, says the CDC.
However, data posted by the CDC on December 8, 2022, indicates the effectiveness of one JYNNEOS dose was 37%, two doses were 69%, and the duration of immunity is unknown.
The CDC announced on February 22, 2023, the Interim Clinical Considerations mpox vaccination should continue to be offered to people with the highest potential for exposure to mpox.
Furthermore, the CDC expects additional mpox cases among previously vaccinated people to occur during the spring and summer seasons in 2023 as people gather for festivals and other events.
Additional mpox outbreak news is posted by Precision Vaccinations.

VBI Vaccines Inc. today announced the expansion of its 3-antigen hepatitis B vaccine and increased sales. PreHevbrio™ is the only 3-antigen hepatitis B vaccine available as of May 15, 2023, comprised of the three surface antigens of the hepatitis B virus – S, pre-S1, and pre-S2.
This innovative vaccine is approved for use in the U.S., European Union/European Economic Area, United Kingdom, Canada, and Israel under the brand names PreHevbrio™ (U.S./Canada), PreHevbri® (EU/EEA/UK), and Sci-B-Vac® (Israel).
In the U.S., PreHevbrio is now available for purchase at six retail pharmacy chains, including Costco, RiteAid, Walmart, and three of the top 10 regional retail pharmacy networks, as well as through the U.S. Department of Veterans Affairs, Federal Bureau of Prisons, and at certain military treatment facilities.
PreHevbrio increased the total number of customer orders in Q1 2023 by 170% compared to Q4 2022.
In a press release, Jeff Baxter, VBI's President, and CEO, commented, "We continue to make good progress across all three endeavors, and I am especially excited to note the increase in the use of PreHevbrio in the U.S."
"With an ever-expanding access and distribution network in place and our focus on commercial execution, we expect to see this momentum continue throughout 2023 and beyond."
Furthermore, the Fance-based specialty vaccine company Valneva SE distributes PreHevbri throughout select European countries, which includes the United Kingdom, Sweden, Norway, Denmark, Finland, Belgium, and the Netherlands.

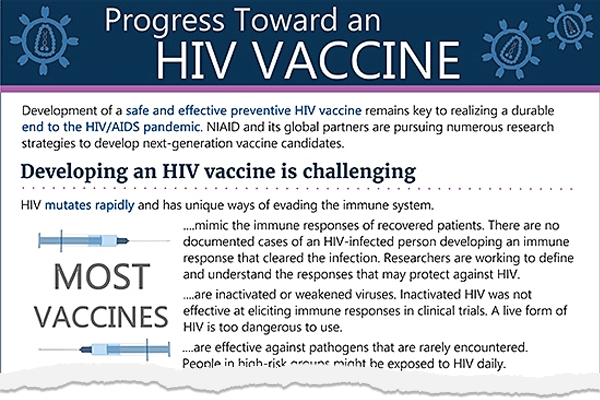
Theratechnologies Inc. recently presented data from a study in which the use of Trogarzo® (ibalizumab), a monoclonal antibody antiretroviral therapy (ART), was associated with favorable virologic outcomes compared to non-ibalizumab regimens used in routine care in heavily treatment-experienced people with HIV.
In the new study, using ibalizumab resulted in a statistically significant doubling of the likelihood of viral undetectability and a much longer duration of undetectability and viral suppression compared to a real-world, non-ibalizumab control group from the Observational Pharmaco-Epidemiology Research & Analysis (OPERA®) database.
The ibalizumab study is thought to be the first matching-adjusted indirect treatment comparison study in HIV, an approach designed to facilitate a closely matched comparison from a synthesized, real-world population when randomization to a control arm would be impractical or unethical.
Christian Marsolais, Ph.D., Senior Vice President and Chief Medical Officer of Theratechnologies, stated in a press release on May 4, 2023, “This is the largest dataset and longest follow-up for Trogarzo® since our Phase 3 study, and reinforces its importance in a patient population that historically has had limited novel treatment options.”
The study evaluated data from 76 participants in two clinical trials (Phase 2b and Phase 3) who received 800 mg of ibalizumab every two weeks (treatment arm) and compared those data to outcomes from 65 individuals treated with non-ibalizumab-containing regimens as routine care in the OPERA® cohort (control arm).
Standardized mortality rate weighting ensured a balance between the treatment and control groups regarding baseline age, CD4 cell count, viral load, and susceptibility to specific ART agents.
At 24 weeks, investigators observed a statistically significant doubling of the likelihood of viral undetectability (defined as VL <50 c/mL) in the treatment arm versus the control arm (SMR-weighted hazard ratio [HR]: 1.98; 95% confidence interval [CI]: 1.02, 3.69).
Achievement of viral suppression (defined as VL <200 c/mL) was also more likely with ibalizumab, though this finding did not reach statistical significance (SMR-weighted HR: 1.28; 95% CI: 0.82, 2.06).
Among those who achieved undetectability on ibalizumab, 95% maintained undetectability through the end of follow-up.
Additionally, the exact significance emerged for maintaining viral suppression, which was 18 times lower for real-world non-ibalizumab regimens.
For both durability analyses, confidence intervals were wide but statistically significant (SMR-weighted HR: 18.36; 95% CI: 2.48, 135.68).
Note: Monoclonal antibody antiretroviral therapy is not an HIV vaccine candidate.