Search API
Pfizer Inc. today announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO™, the company’s bivalent Respiratory Syncytial Virus (RSV) prefusion F (RSVpreF) vaccine.
This FDA approval on May 31, 2023, prevents lower respiratory tract disease caused by RSV in individuals 60 years and older.
On May 3, 2023, the FDA approved the initial RSV vaccine, Arexvy™, which GSK produces.
According to statements, both RSV vaccines could be available for seniors in late 2023.
Other RSV vaccine candidates, including vaccines for pregnant women, are conducting late-stage clinical trials as of June 1, 2023.
The U.S. Centers for Disease Control and Prevention published a report on April 7, 2023, that indicated the 2022–23 RSV season started later than the 2021–22 season but earlier than the prepandemic seasons, suggesting a return toward prepandemic seasonality.
A study published by the Journal of Infectious Diseases determined that RSV-related fatalities in infants <1 year peaked at one month of age.
Over the 20-year study period, RSV, bronchiolitis, and influenza were listed as the underlying causes of death on 932, 1,046, and 52,293 death certificates, respectively.
Over 95% of these infections in children occur in low- and middle-income countries outside the U.S.

Southern Africa is again weathering a season of cholera, with six countries in the region recording outbreaks in 2023, reported Derick Matsengarwodzi with GAVI.
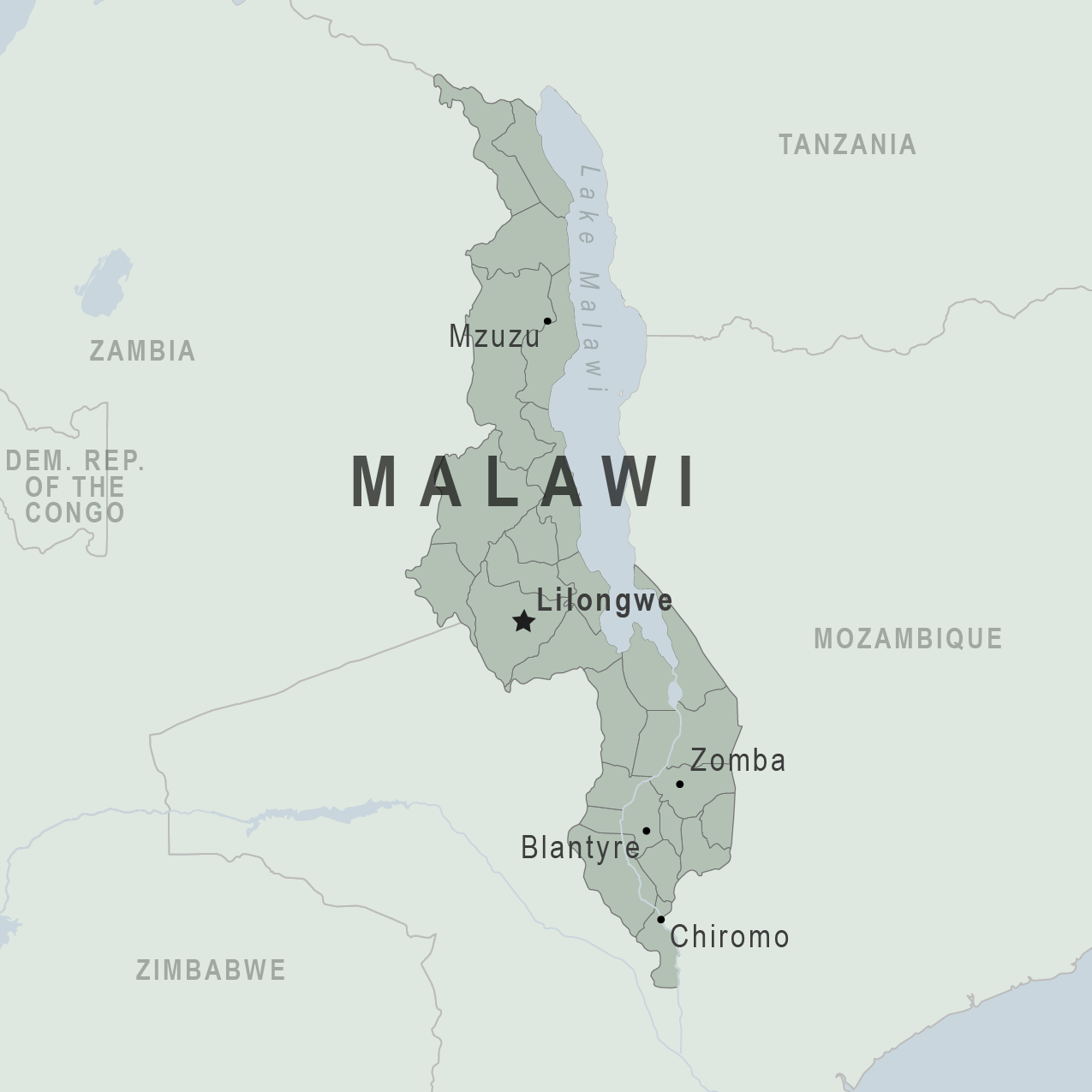
As of May 25, 2023, Malawi is the worst affected, recording 36,943 cases and 1,210 associated deaths between March 2022 and February 2023, according to World Health Organization (WHO).
So far this year, Malawi has received three shipments of oral cholera vaccine (OCV) in response to applications to the Gavi-supported stockpile established in 2013.
The latest 1.4 million OCV dose shipment arrived in Lilongwe in April 2023.
On May 22, 2023, Gavi published a roadmap outlining critical actions needed to ensure the supply of OCV can meet growing demand from countries.
The roadmap describes how these organizations, manufacturers, and countries can work together towards ensuring global OCV supply can support large-scale preventive vaccination by 2026.
"Cholera vaccines have steadily become more available over the past decade, meeting rising country demand," said Dr. Derrick Sim, Managing Director for Vaccine Markets and Health Security at Gavi, in a media release.
"As a result, the good news is we have doses to meet all emergency demand despite the rise in outbreaks, which is expected to continue. But this trend underscores the increasing importance of preventing outbreaks before they occur."
"The ultimate solution to both sustainable OCV supply and cholera control lies in our collective ability to step up our efforts on prevention programs."
As of May 31, 2023, various cholera vaccines have been approved but remain in limited supply.
Note: The U.S. CDC recently issued an Alert Level 2, Practice Enhanced Precautions, regarding polio outbreaks, which includes Malawi.
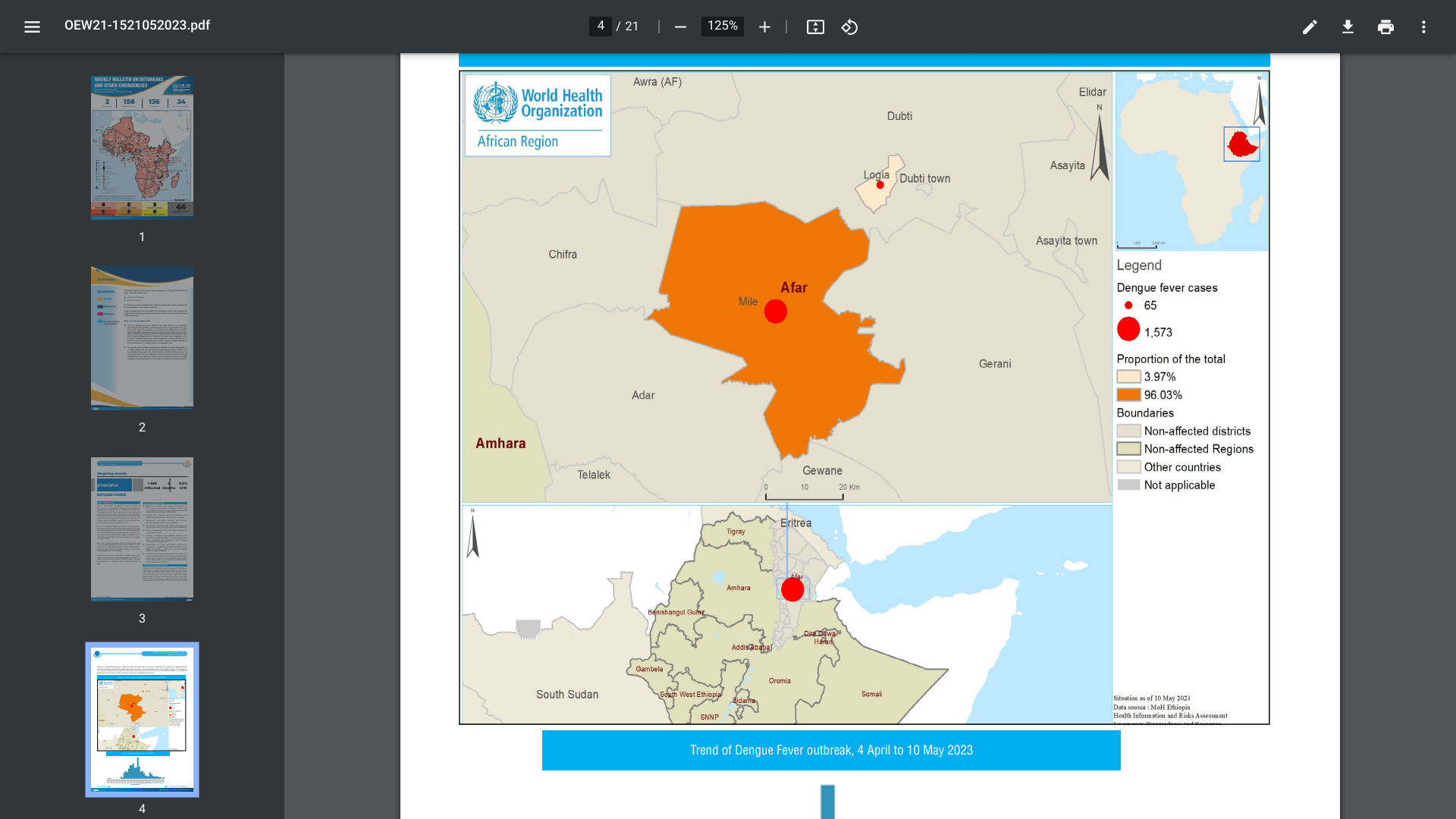
The World Health Organization (WHO) Africa Region recently reported two districts (Logia and Mille) of the Afar region in north-eastern Ethiopia are experiencing a Dengue fever outbreak.
The Mille district has reported the most dengue cases (96%).
As of May 10, 2023, a total of 1 638 suspected and confirmed dengue cases and nine associated deaths (CFR 0.5%) have been reported.
Of the nine suspected deaths reported, four occurred at the health facility and five at the community level.
Ethiopia has had nearly annual outbreaks since 2013, devastating an already fragmented health system, says the WHO. Furthermore, dengue is a vaccine-preventable disease, with two vaccines available in 2023.
Other dengue outbreaks in 2023 are posted at Vax-Before-Travel.
The U.S. CDC Morbidity and Mortality Weekly Report (MMWR) Podcast Briefing, published today, offers an overview of the latest scientific information regarding mpox vaccinations.
This podcast discusses three MMWR reports as of the week of May 15, 2023.
First, although the number of mpox cases has decreased since the peak of the U.S. outbreak in August 2022, the risk for future outbreaks remains.
And clinicians need to be alert for new cases, and people at risk should continue to take prevention measures.
Second, a new report looking at data from 12 U.S. jurisdictions shows Bavarian Nordic JYNNEOS® (MVA-BN) vaccine effectively prevents mpox in people at high risk for mpox.
Third, a study of mpox patients in New York provides evidence that the JYNNEOS vaccine is highly effective against mpox.
However, the CDC and other health agencies in France, South Korea, and Spain previously reported various mpox breakthrough cases in 2023.

EL PAIS recently reported two patients were diagnosed for the second time with mpox in Spain.
On May 26, 2023, the Vall d'Hebron (Barcelona) and Ramón y Cajal (Madrid) hospitals confirmed two patients had been reinfected with mpox.
EL PAIS reported these are the first mpox reinfection cases in Spain.
According to the Carlos III Health Institute, there have been 56 mpox cases in Spain since the beginning of 2023.
Previous reports in 2023 confirmed secondary mpox cases in Chicago, Il, Paris, France, and Seoul, South Korea.

Immorna today announced that the first subject had been dosed in the Company's First-In-Human Phase 1 multi-center study of JCXH-105, a self-replicating RNA (srRNA) vaccine being developed for the prevention of Shingles.
The U.S. Food and Drug Administration (FDA) cleared its investigational new drug application on January 9, 2023, to conduct a Phase 1 multi-center study of JCXH-105.
NgocDiep Le, M.D., Ph.D., Global Chief Medical Officer of Immorna, commented in a press release on May 30, 2023, "If proven successful in clinical studies, JCXH-105 may become a valuable alternative to current standard-of-care to meet the large world-wide medical need for Shingles prevention."
"Due to its self-replicating nature, JCXH-105 may be effective at a significantly reduced dose level compared to non-replicating conventional mRNA vaccines and thereby may cause less reactogenicity and substantially reduce the cost of production."
"In addition, due to the synthetic nature of all JCXH-105 vaccine components, there are no raw material limitations or production bottlenecks."
This Phase 1 study is a randomized, double-blinded, multi-center, active-controlled study to assess the safety, immunogenicity, and determine the Recommended Phase 2 Dose for JCXH-105 for seniors.
In this study, JCXH-105 will be compared to GSK's U.S. FDA-approved Shingrix® vaccine.
Other shingles vaccine development news is posted by Precision Vaccinations.