Search API
Puerto Rico's Department of Health Arboviral Disease recently reported increased dengue and Zika cases.
As of May 25, 2023, the data for week #19 indicates 305 dengue and 25 Zika probable cases so far this year.
While no Zika vaccines are available, Puerto Rico is evaluating the Dengvaxia® vaccine in the greater San Juan area.
The U.S. CDC has scheduled a vaccine committee to discuss Puerto Rico's test results and review the new QDENGA® vaccine.
Qdenga has been approved for use in Argentina, Brazil, Indonesia, the European Union, and the U.K. as of May 2023.

Biofabri and IAVI recently announced signing an agreement for the end-to-end development of tuberculosis (TB) vaccine candidate MTBVAC. This agreement provides a framework for the future collaboration that the partners first announced in 2021.
After securing sufficient funding, IAVI plans to begin an efficacy clinical trial in 2024.
MTBVAC is a highly promising vaccine candidate that has the potential to be used as an alternative to BCG vaccination in infants and for the prevention of TB disease in adolescents and adults.
"The world urgently needs a new, effective vaccine that can prevent TB disease in adults, adolescents, and infants," said Dr. Mark Feinberg, president, and CEO of IAVI, in a press release on May 17, 2023.
"We are honored to work with Biofabri and our other collaborators to advance MTBVAC."
"In addition, we are actively seeking the support of global health funders and other partners, public and private, to ensure that this promising vaccine candidate has the potential to be part of a solution to ending the TB epidemic."
Should MTBVAC be safe and efficacious, Biofabri will ensure that the TB vaccine is manufactured and supplied in sufficient quantities globally and is accessible at affordable prices in low- and middle-income countries.
Precision Vaccinations post other TB vaccine and outbreak news.

The U.S. Food and Drug Administration (FDA) today approved Pfizer Inc.'s oral antiviral Paxlovid™ for treating mild-to-moderate COVID-19 in adults at high risk for progression to severe COVID-19, including hospitalization or death.
Paxlovid is the fourth drug, but the first oral antiviral approved to treat COVID-19 in adults.
Paxlovid manufactured and packaged under the emergency use authorization (EUA) and distributed by the U.S. Department of Health and Human Services will continue to be available to ensure continued access for adults, as well as treatment of eligible children ages 12-18 who are not covered by the FDA's approval on May 25, 2023.
"While the pandemic has been challenging for all of us, we have made great progress mitigating the impact of COVID-19 on our lives," said Patrizia Cavazzoni, M.D., director for the FDA's Center for Drug Evaluation and Research, in today's press release.
"Today's approval demonstrates that Paxlovid has met the agency's rigorous standards for safety and effectiveness and that it remains an important treatment option for people at high risk for progression to severe COVID-19, including those with prior immunity."
"The FDA remains committed to working with sponsors to facilitate the development of new prevention and treatment options for COVID-19."
Paxlovid is not approved or authorized for use as a pre-exposure or post-exposure prophylaxis to prevent COVID-19.

The U.S. State Department announced on May 22, 2023, the U.S. Embassy in Khartoum has suspended its operations and issued a Level 4, Do not travel to Sudan due to extensive civil unrest.
As a result, the embassy cannot provide routine or emergency services to U.S. citizens in Sudan.
Currently, there are no U.S. consular officers in Sudan.
However, consular services are available in neighboring countries for those who depart independently.
In addition, the U.S. government is providing information for U.S. citizens in Sudan, including exit options.
Specifically, U.S. citizens should not visit the Hotel Coral in Port Sudan or the Fenti Golf in Khartoum. The U.S. government is no longer present to assist in these locations.
We understand that border crossings into neighboring countries, such as Egypt, may be possible.
However, if you can travel to a border crossing and believe it is safe, please be aware that wait times and conditions at crossing points vary widely and could change quickly, wrote the State Department.
And enroll in the Smart Traveler Program to receive security updates.
U.S. citizens in Sudan who need assistance should contact the closest U.S. embassy or consulate.

The U.S. CDC's Vessel Sanitation Program (VSP) requires cruise ships to log and report the number of passengers and crew who say they have symptoms of gastrointestinal illness.
As of May 24, 2023, the VSP has reported 12 gastrointestinal outbreaks on cruise ships.
Data are from ship surveillance reports and CDC-led investigations.
People often associate cruise ships with acute gastrointestinal illnesses such as norovirus. However, acute gastrointestinal illness is relatively infrequent on cruise ships.
Norovirus is a very contagious virus that causes 19 to 21 million illnesses annually in the U.S.
You can get norovirus from an infected person, contaminated food or water, or by touching contaminated surfaces. The virus causes your stomach or intestines or both to get inflamed (acute gastroenteritis).
This leads you to have stomach pain, nausea, and diarrhea and to throw up, says the CDC.
According to a CDC norovirus tracking network, there were 735 outbreaks reported from August through April 23.
There were 907 during the same period of the 2021-2022 norovirus season.
While vaccines against norovirus diseases are in demand, noroviruses' genetic and antigenic diversity presents challenges. As a result, as of May 2023, no norovirus vaccines are authorized.

Prensa Latina recently reported Cuba's COVID-19 vaccines, Soberana 02, Soberana Plus, and Abdala, received the sanitary registration granted by the Center for State Control of Medicines, Medical Equipment, and Devices for their proven efficacy.
The experts and scientists ratified the safety that characterizes Cuba's COVID-19 vaccines, firstly due to the very nature of the technological platforms used and secondly, because they have high thermo-stability.
Unlike others requiring special storage conditions, Cuba's vaccines can be stored between two and eight degrees Celsius at freezing temperatures.
During the clinical studies, vaccine efficacy was higher than 90%.
As of May 24, 203, Cuba is among the world's top 10 countries with most citizens immunized against the SARS-CoV-2 coronavirus, which raises the level of protection to more than 90% of Cuban inhabitants.

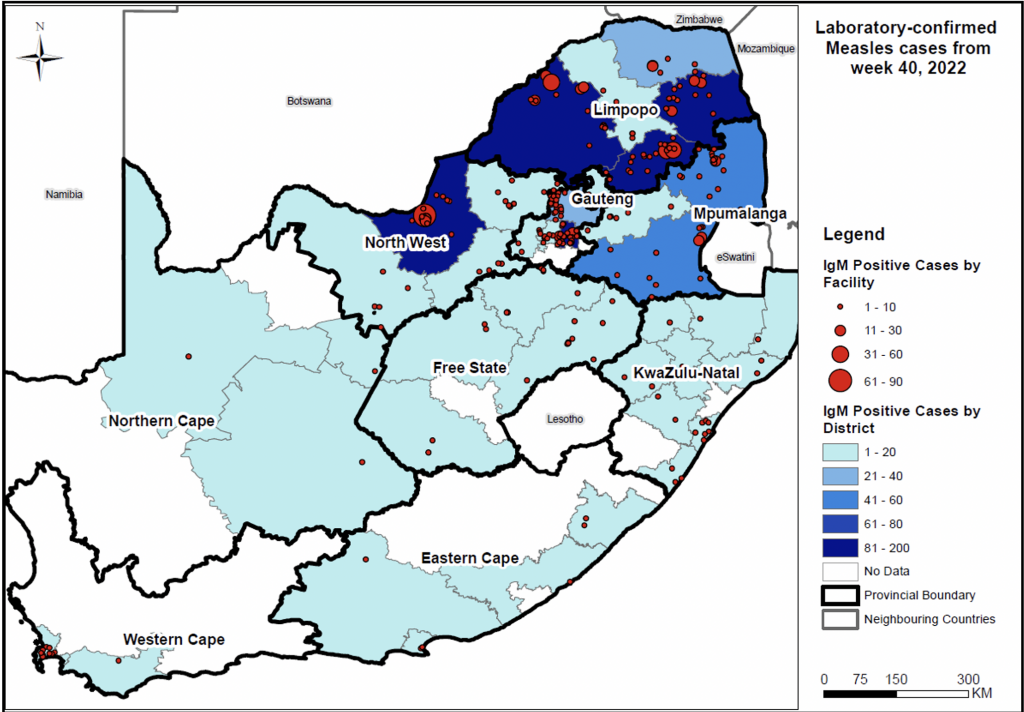
The South African National Institute for Communicable Diseases recently confirmed a measles outbreak had been declared in all the provinces in South Africa except for the Eastern Cape since late 2022.
In week #18, seven new measles cases were reported from Limpopo province.
In total, 1004 measles cases have been reported as of May 18, 2023.
The measles strain detected in Limpopo province and North West province is genotype D8, similar to the strain in Zimbabwe in the 2022 outbreak.
Globally, measles outbreaks are occurring in numerous countries.
Therefore, the U.S. CDC suggests ensuring international travelers are protected before departure.
Measles is a vaccine-preventable disease, with various vaccines offered in most countries.