Search API
Following the confirmation of an outbreak of Sudan virus disease in the Republic of Uganda, the World Health Organization (WHO) announced it is mobilizing efforts to support the national health authorities in containing a potential outbreak in Kampala.
The identification of the case in a densely populated urban requires a rapid and intense response, says the WHO.
As of January 30, 2025, a nurse from Mulago National Referral Hospital in the capital, Kampala, a city with about 1.8 million residents, has been reported with this disease.
A total of 45 contacts, including health workers and family members of the confirmed case (deceased), have been identified and are currently under close monitoring. No other health workers or patients have shown symptoms of the disease.
“We welcome the prompt declaration of this outbreak, and as a comprehensive response is being established, we are supporting the government and partners to scale up measures to quickly identify cases, isolate and provide care, curb the spread of the virus, and protect the population,” said Dr Matshidiso Moeti, WHO Regional Director for Africa, in a press release.
Eight previous outbreaks of the Sudan virus disease have occurred, five in Uganda and three in Sudan. Uganda last reported an outbreak in 2022.
Sudan virus disease is a severe, often fatal illness affecting humans and other primates. It is caused by Orthoebolavirus Sudanese (Sudan virus), a viral species belonging to the same genus as the virus that causes Ebola virus disease.
Case fatality rates of Sudan virus disease have varied from 41% to 100% in past outbreaks.
While no licensed vaccines for the Sudan virus disease exist, the WHO coordinates with developers to deploy candidate vaccines and other public health measures.
The WHO stated that experimental vaccines would be deployed once all administrative and regulatory approvals were obtained.
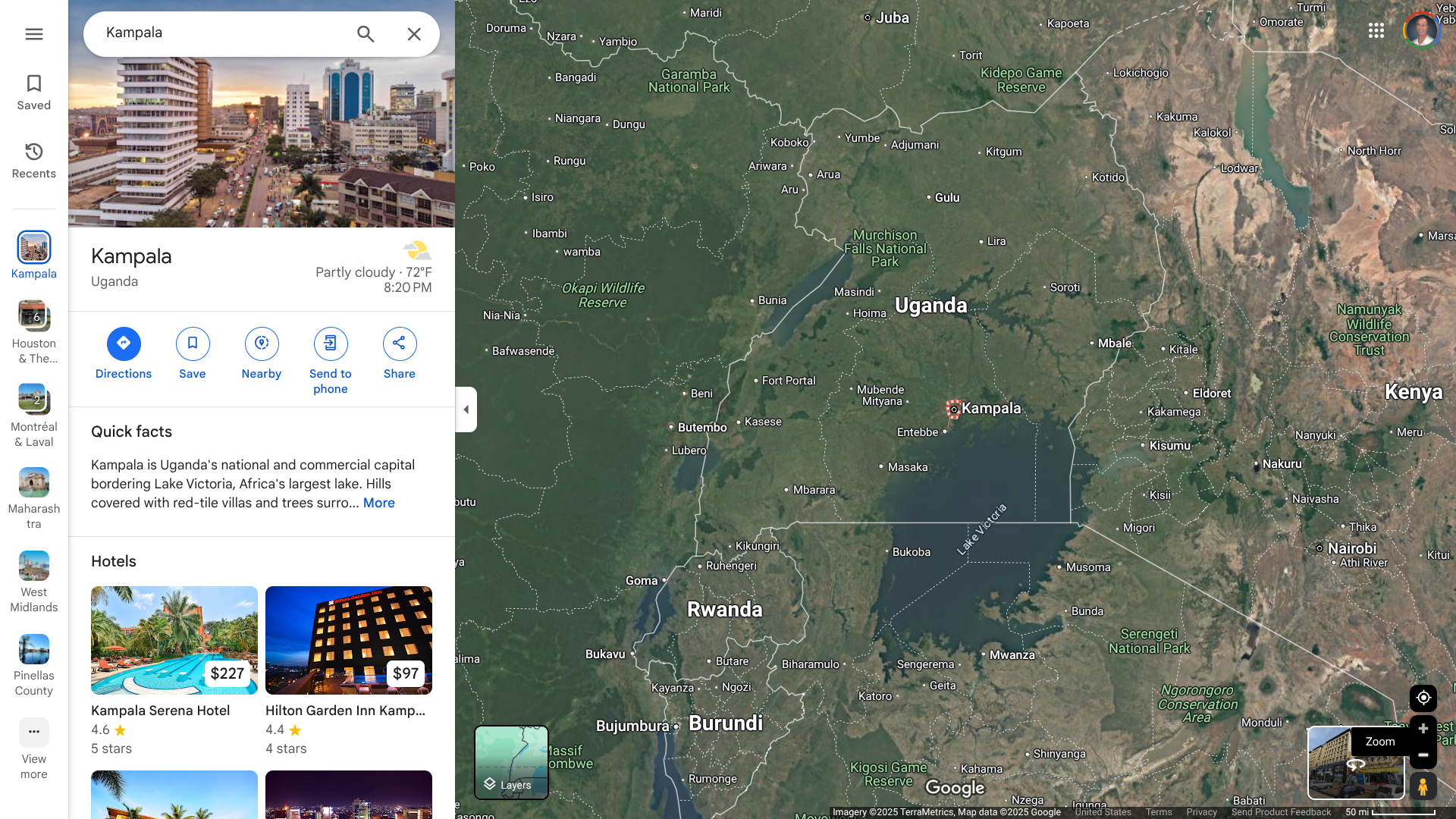
The World Health Organization's Disease Outbreak News recently reported a fatal case of Chapare hemorrhagic fever (CHHF) from the La Paz Department in the Plurinational State of Bolivia.
As of January 20, 2025, no secondary cases have been reported.
The WHO defines Chapare hemorrhagic fever as an acute viral illness caused by the Chapare virus. The rodent-borne Chapare virus is an Arenavirus that can cause hemorrhagic fevers like Ebolaviruses.
Initially identified in Cochabamba in 2003, five documented outbreaks have occurred within Bolivia.
The most recent outbreak occurred in 2024, with one laboratory-confirmed case within the La Paz Department. This area in Bolivia, which has a population of about 3 million, is a neighbor of Peru.
As of January 22, 2025, the WHO says there is no significant risk of the disease spreading internationally. Person-to-person transmission of the Chapare virus is possible but remains rare in the general population.
The U.S. CDC says CHHF is a rare, deadly viral disease. About 20% to 60% of people with the disease die.
Furthermore, there are no treatments or preventive vaccines available for CHHF.
When visiting Bolivia in 2025, the CDC recommends several travel vaccinations, such as chikungunya and yellow fever. These vaccines are offered at many travel clinics and pharmacies in the U.S.
Emergent BioSolutions Inc. today announced that the Biomedical Advanced Research and Development Authority (BARDA) executed a contract modification for the second option period, valued at approximately $16.7 million.
This option is part of Emergent's existing 10-year contract with BARDA for the advanced development and procurement of Ebanga™, which has a maximum value of $704 million.
This modification will validate the drug product process and analytical testing and ensure long-term stability for Ebanga, which is indicated for treating infection caused by the Zaire Ebola virus.
Ebanga (ansuvimab-zykl) is a Zaire ebolavirus glycoprotein-directed human monoclonal antibody indicated for treating infection caused by Zaire ebolavirus in adult and pediatric patients.
As of 2025, the U.S. Department of Homeland Security has determined that Ebolavirus disease (EVD) threatens national health security. To augment the government's response capability, BARDA is pursuing the advanced development, licensure, and procurement of therapeutics that can be deployed in EVD outbreaks.
"We are delighted our continued collaboration with BARDA is advancing Ebanga development toward supplying treatment and ensuring communities are prepared against Ebola (outbreaks)," said Simon Lowry, M.D., chief medical officer, head of research and development, Emergent, in a press release on January 13, 2025.
"Ebola is a devastating infectious illness with limited treatment options."
Ebanga is not a preventive vaccine.
As of early 2025, Merck's U.S. FDA-approved Ervebo® (rVSV-ZEBOV) vaccine was licensed in the U.S., the U.K., the European Union, Canada, and various countries. Recently, Sierra Leone became the first country in Africa to launch a preventive Ebola vaccination campaign targeting health workers.
Ervebo is not commercially available in the U.S.
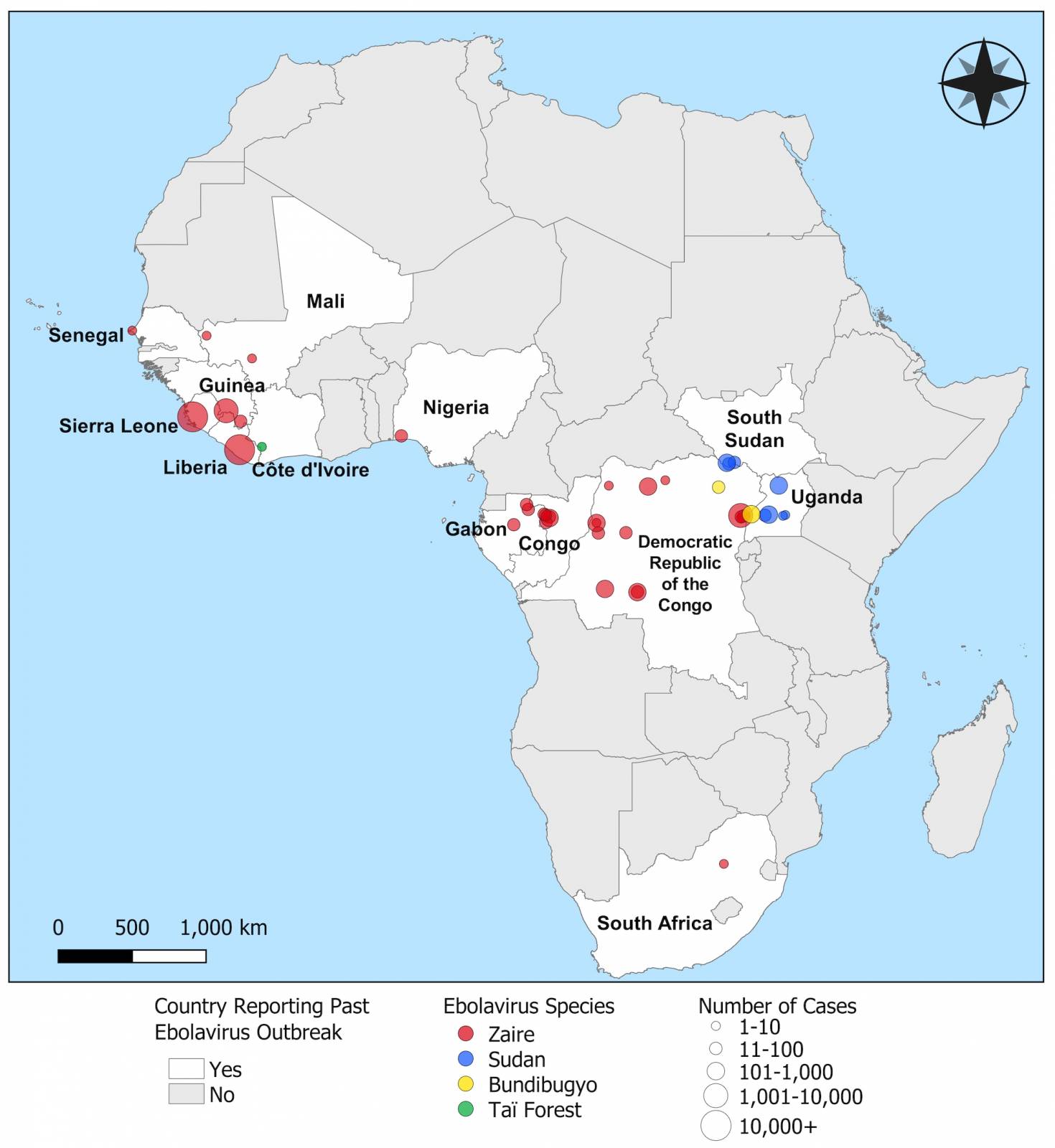
Orthoebolavirus zairense (EVD) is severe and often fatal, with case fatality rates ranging from 25% to 90%, and is transmitted via bodily fluids, zoonotic transmission, or contact with contaminated surfaces.
According to the World Health Organization, more than 30 EVD outbreaks have been reported. The initial Zaire Ebolavirus case was confirmed in 1976 in a village near the Ebola River.
As of 2025, no active U.S. CDC Travel Health Notice is focused on Ebola outbreaks in Africa.

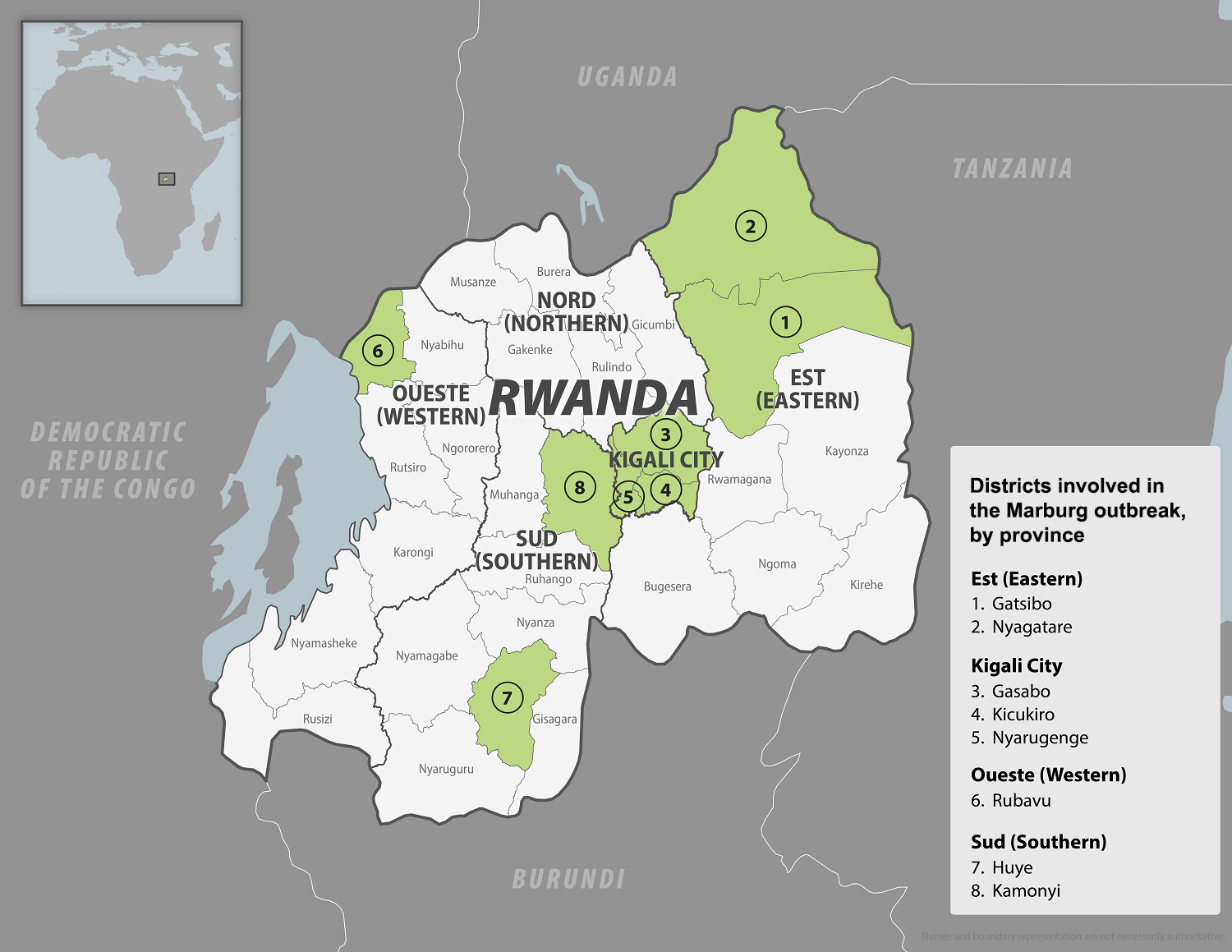
After 42 days without detecting a new case, the World Health Organization (WHO) announced that the Marburg Virus Disease (MVD) outbreak in the Republic of Rwanda had ended.
The outbreak, confirmed in late September 2024, was the first Marburg Virus Disease outbreak Rwanda has experienced. A total of 66 confirmed cases and 15 deaths (23%) were recorded. Almost 80% of the cases were among infected health workers while providing clinical care to their colleagues and other patients.
"The robust response by Rwanda shows how committed leadership, concerted efforts by partners, and a strong health system are crucial in addressing public health emergencies, saving and protecting lives, as well as safeguarding the health of individuals and communities," said Dr. Brian Chirombo, WHO Representative in Rwanda, in a media release issued on On December 20, 2024.
The virus which causes Marburg is in the same family as the virus that causes Ebola Virus Disease. Marburg virus is transmitted to people from fruit bats and spreads among humans through direct contact with the bodily fluids of infected people, surfaces, and materials.
In October 2024, the Sabin Vaccine Institute announced it dispatched investigational vaccine doses for a randomized clinical trial targeting Rwanda's outbreak.
As of December 2024, there are no approved Marburg virus vaccines.
A decade after the deadliest Ebola virus disease (EVD) outbreak in history, Sierra Leone will become the first country to launch a preventive Ebola vaccination campaign targeting 20,000 frontline workers in all 16 districts across the country.
A single dose of the Ebola vaccine Ervebo will be administered to health care professionals, frontline workers, and first responders such as motorbike riders/ambulance drivers, traditional healers, religious leaders, security forces, and others who are at high risk of being exposed to EVD.
"Protecting our frontline workers is vital to our National Health Security Plan, ensuring preparedness and resilience against future health threats. This is an investment in the safety of our people and a healthier Sierra Leone," said Dr Austin Demby, Minister of Health, Sierra Leone, in a press release on December 4, 2024.
This preventive vaccination campaign comes after the deadly 2014–2016 Ebola outbreak was declared a Public Health Emergency of International Concern. That outbreak resulted in more than 11,000 deaths in 10 countries around the world.
Of these, Sierra Leone reported close to 4,000 deaths.
The first cases of EVD were detected in Sudan and the Democratic Republic of the Congo in 1976. The initial Zaire Ebolavirus outbreak was confirmed in a village near the Ebola River.
Merck Ervebo® Ebola Vaccine (rVSV-ZEBOV-GP, rVSV-ZEBOV, v920) is a live, recombinant, replication-competent Orthoebolavirus zairense vaccine that the U.S. FDA approved on December 19, 2019. Orthoebolaviruses are a group of four viruses that cause EVD.
Vaccines for the Sierra Leone campaign are sourced from the Gavi-funded global vaccine stockpile administered by the International Coordinating Group on Vaccine Provision.
Ervebo is not commercially available in the U.S.
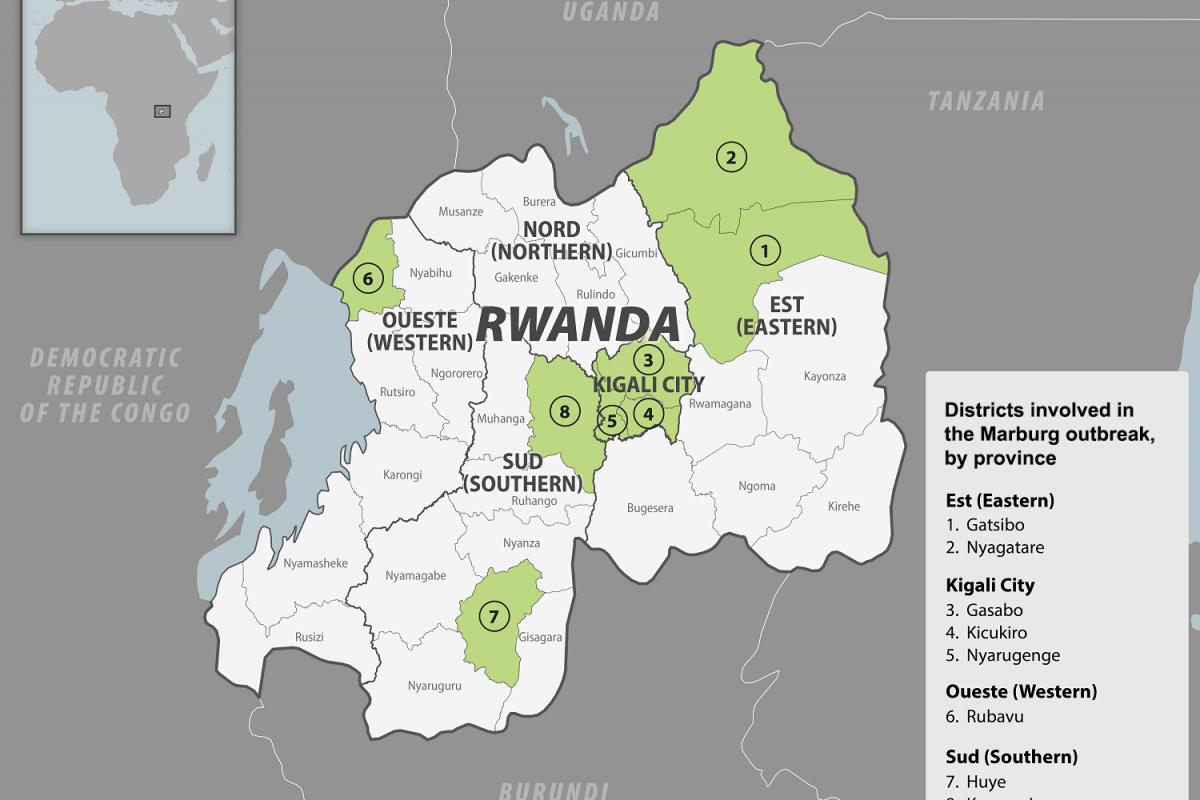
The WHO Africa recently announced that the Republic of Rwanda had discharged the last Marburg virus disease (MVD) patient, kicking off the mandatory 42-day countdown to declare the outbreak's end.
As of November 9, 2024, a total of 66 MVD cases and 15 deaths have been recorded during the outbreak, which was declared on September 27, 2024. Health workers, who constitute almost 80% of the cases, primarily became infected while providing emergency care to their colleagues and patients.
"This outbreak demonstrates that with the best available treatment, recovery is possible, and contributions to science can be made," said Dr Sabin Nsanzimana, Rwanda's Minister of Health, in a press release.
The World Health Organization published the Marburg vaccine development landscape on February 13, 2023. As of November 2024, no approved MVD vaccines exist.
Marburg is a highly virulent virus with a fatality ratio of up to 88%, and it was initially detected in Germany in 1967 following a lab incident. The virus belongs to the same family as the Ebola virus. Illness begins abruptly with high fever, severe headache, and malaise, and many patients develop severe hemorrhagic symptoms within seven days.
Currently, the U.S. CDC's Level 3—Reconsider Nonessential Travel Advisory remains active. The CDC recommends reconsidering nonessential travel to Rwanda, which is experiencing an outbreak of Marburg.
Bavarian Nordic A/S today announced the initiation of a clinical study of the MVA-BN® (JYNNEOS) mpox/smallpox vaccine in children 2 to 11 years of age, partially funded with $6.5 million from the Coalition for Epidemic Preparedness Innovations (CEPI).
The phase 2 study is currently enrolling children in the Democratic Republic of Congo, with plans to include sites in Uganda. Results from this study could support an extension of the current approval of MVA-BN to include young children.
Last month, the WHO prequalified MVA-BN for adolescents 12 to 17 years of age, adopting the recent approval from the European Medicines Agency (EMA) for this age group.
While this study represents the first investigation of MVA-BN as a mpox/smallpox vaccine for younger children, a recombinant version of MVA-BN (Mvabea®) was approved by EMA in 2020 as part of a prime-boost vaccine regimen or the prevention of disease caused by Zaire Ebolavirus.
Paul Chaplin, President and CEO of Bavarian Nordic, said in a press release, “Following the recent approval of MVA-BN for adolescents, we are pleased to initiate this study, which could provide additional data to extend the indication to include children. We thank CEPI and our partners in Africa for their support of this important work.”
From an access perspective, Bavarian Nordic announced an agreement with UNICEF on September 26, 2024, to supply 1 million doses of the MVA-BN® mpox vaccine for African countries impacted by the ongoing mpox outbreak.
As of late October 2024, there have been about 2,230 clade 2 mpox cases reported to the U.S. CDC this year.
The JYNNEOS vaccine is commercially available in the United States at various clinics and pharmacies.

RedHill Biopharma today announced that it had received a contract with the U.S. Biomedical Advanced Research and Development Authority (BARDA) to advance the development of opaganib, a small-molecule treatment for Ebolavirus.
This novel, potentially broad-acting drug has shown mutation-resistant antiviral and anti-inflammatory activity, likely to counteract the vascular impacts of Ebola infection.
In a press release on October 14, 2024, the company stated that it is pursuing an animal-rule pathway for potential approval for this Ebola treatment candidate. This process is used when human clinical trials are not ethical or feasible.
Guy Goldberg, RedHill's Chief Business Officer, commented, "Currently, only Inmazeb™, a combination of three monoclonal antibodies, and Ebanga™, a single monoclonal antibody, are FDA-approved to treat Ebola infections. As such, there is an urgent need for additional effective and easy-to-distribute and administer therapies (during an outbreak)."
While there are approved Zaire Ebola vaccines and therapeutics available in 2024, previous outbreaks have highlighted significant logistical challenges that exist in managing Ebola outbreaks.
As of October 2024, more than 30 Ebola outbreaks have been reported in Africa. The initial Zaire Ebolavirus case was confirmed in 1976 in a village near the Ebola River in Africa, and the virus's origins remain enigmatic in 2024.