Search API
SK bioscience today announced positive results from its Phase II clinical trials in infants of its 21-valent pneumococcal conjugate vaccine candidate, 'GBP410' (SP0202), evaluating its safety and immunogenicity.
Given that GBP410 includes 21 serotypes, it is anticipated to offer broader serotype coverage than the existing pneumococcal conjugate vaccines.
The Phase II study demonstrated comparable immunogenicity of GBP410 compared to the control vaccine, following the primary vaccination at 2, 4, and 6 months of age as well as the booster vaccination for ages of 12 to 15 months.
The data also showed a well-tolerated safety profile, with a similar reactogenicity profile to the control vaccine and no vaccine-related serious adverse events.
Furthermore, GBP410 did not interfere with the immunogenicity and safety profile of the co-administered recommended pediatric vaccines, such as tetanus, diphtheria, pertussis, polio, and Haemophilus influenzae type b vaccines.
Based on the positive safety and immunogenicity data from the Phase II clinical trial, SK bioscience and its development partner Sanofi plan to start Phase III in H1 2024, expecting to secure the final data in 2027.
In preparation for the commercialization of GBP410, SK bioscience intends to enter the U.S. and European markets with Sanofi by making significant investments in manufacturing facilities.
Jean-Francois Toussaint, Global Head of Vaccines R&D at Sanofi, said in a press release on June 29, 2023, "We are pleased with our very productive partnership with SK bioscience as we work to raise the bar in pneumococcal disease."
"With an innovative carrier that breaks the glass ceiling of serotype compositions, our 21-valent pneumococcal conjugate vaccine is designed to offer expanded protection against this devastating disease."
"We believe that today's results offer us a strong path to Phase 3 and then to licensure."

As the new flu season approaches this fall, influenza viruses from last season continue negatively impacting people.
On June 30, 2023, the U.S. Centers for Disease Control and Prevention (CDC) published the Weekly U.S. Influenza Surveillance Report confirmed respiratory illness, often called influenza-like illness, severely impacts people.
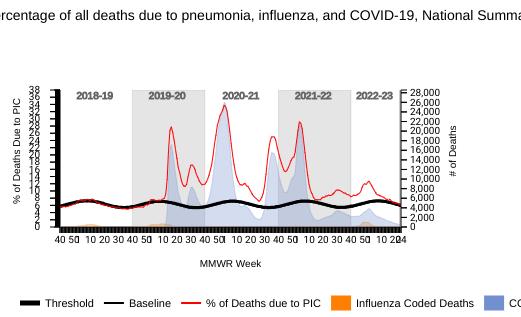
According to National Center for Health Statistics Mortality Surveillance data on June 29, 2023, 6.2% of the deaths during week #25 were due to pneumonia, influenza, and/or COVID-19 (PIC).
Among the 1,342 PIC deaths reported for this week, 240 had COVID-19 listed as an underlying or contributing cause of death on the death certificate, and eight listed as influenza.
The majority of deaths were related to pneumonia.
Furthermore, one additional influenza-associated pediatric death occurring during the 2022-2023 season was reported to CDC during week 25.
Throughout the 2022-2023 flu season, the total number of pediatric deaths totaled 160.
In the 2019-2020 flu season, 199 children died from influenza infections.
With the new flu season starting soon, the CDC recently announced good news regarding vaccines.
The U.S. CDC adopted the 2023-2024 recommendations on annual influenza vaccination for everyone six months and older on June 27, 2023.
Additionally, flu vaccinations in July and August are not recommended for most people, but there are several considerations for specific groups.
The U.S. Centers for Disease Control and Prevention (CDC) recently stated dengue is an ongoing risk in many parts of Asia and the Pacific Islands.
On June 28, 2023, the CDC reissued a Level 1 - Practice Usual Precautions, Travel Health Advisory that revealed the nine countries reported higher-than-usual dengue cases.
And travelers visiting these countries may be at increased risk.
Dengue is a vaccine-preventable disease caused by a virus spread through mosquito bites. The disease can take up to two weeks to develop, with illness generally lasting less than a week.
The CDC says severe health effects include bleeding, shock, organ failure, and death.
As of June 30, 2023, dengue outbreaks have been reported in Florida, Mexico, Costa Rica, and various Central and South American countries.

The Chicago Department of Public Health (CDPH) reconfirmed on June 27, 2023, it is investigating a recent increase in mpox cases among Chicago residents.
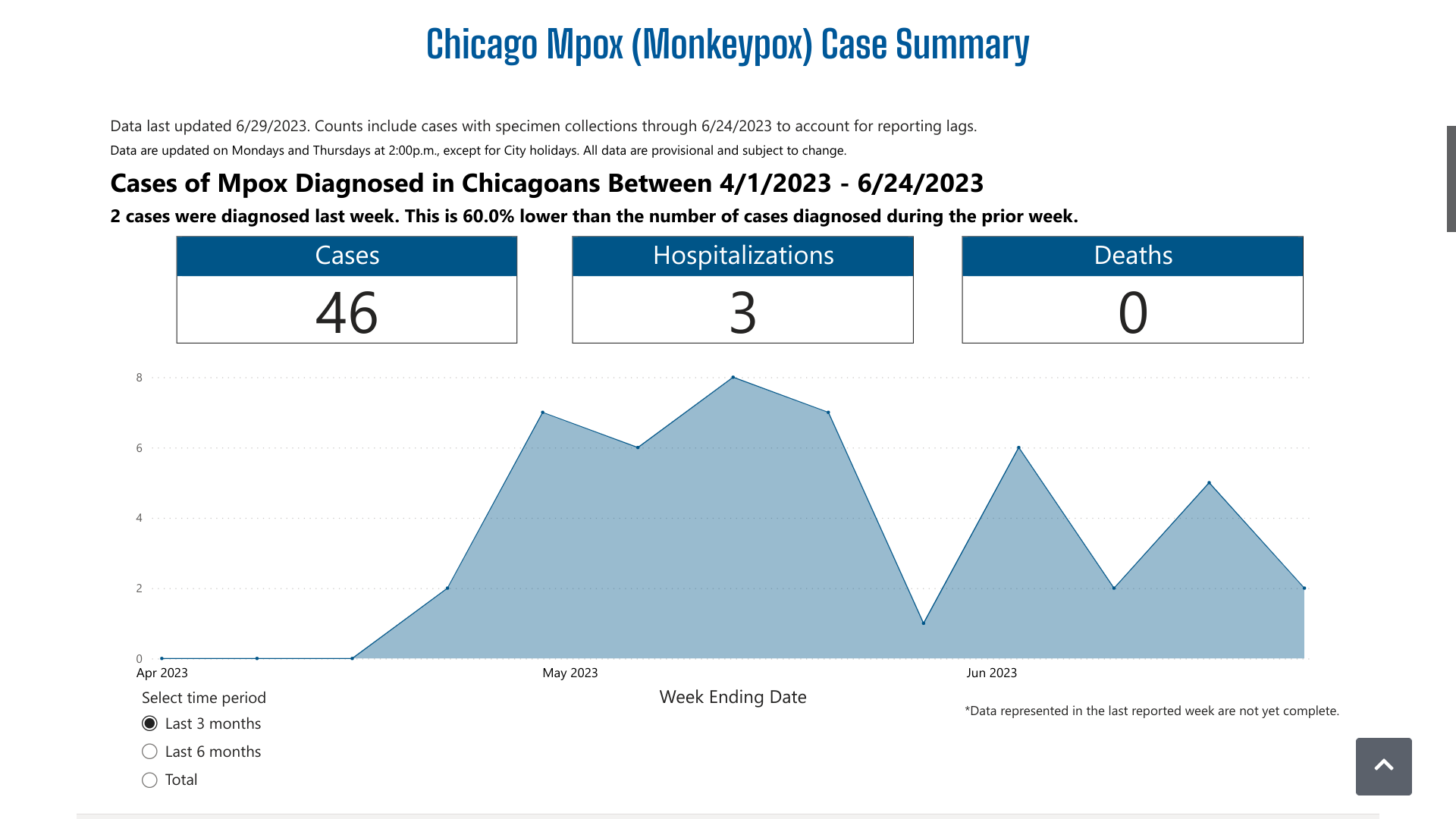
Between June 4 and June 22, 2023, there were six additional mpox cases reported.
The CDPH's dashboard indicates over the past three months, there have been 46 mpox cases and three related hospitalizations. And Chicago did not disclose the number of vaccine-breakthrough cases reported.
From a prevention perspective, 146 people were vaccinated in Chicago last week with the JYNNEOS® (MVA-BN) vaccine.
Since May 2022, 49,351 doses have been administered.
Chicagions with mpox questions can contact the HIV/STI Resource Hub at 844-482-4040 or the CDPH Call Center at 312-746-4835.
Throughout Illinois, including CDPH's data, 1,488 mpox cases have been reported during the global outbreak.
As of June 28, 2023, the U.S. CDC reported 30,531 mpox cases and 43 related fatalities since May 2022.
While Bavarian Nordic's vaccine has been the primary mpox vaccine offered in the U.S., a Cincinnati-based firm Blue Water Biotech, Inc., announced on June 28, 2023, preliminary preclinical data supporting the use of its norovirus shell and protrusion virus-like particle platform to develop a novel mpox vaccine candidate.
The World Health Organization (WHO) reported this week respiratory syncytial virus RSV activity was generally low worldwide except in Australia and a few countries in the Region of the Americas.
And as of June 26, 2023, RSV activity increased in a few tropical and temperate South American countries.
Furthermore, in the United Kingdom, the RSV detection rate among children under five years of age remained at a low level in June 2023.
In the U.S., Florida's RSV season is longer than the rest of the country, says the U.S. Centers for Disease Control and Prevention (CDC). For this reason, Florida is a good bellwether state for the forthcoming RSV season.
According to the Florida Department of Health, RSV activity was low in all five regions, with no outbreaks but an increased positivity rate as of June 24, 2023.
From a prevention perspective, the CDC approved RSV vaccines for people ages 60 and older, using shared Clinical Decision-Making.
This means these individuals may receive a single dose of the RSV vaccine based on discussions with their healthcare provider about whether RSV vaccination is right for them.

The U.S. Centers for Disease Control and Prevention (C.D.C.) today announced the current C.D.C. Director adopted the 2023-2024 Advisory Committee on Immunization Practices' (ACIP) recommendations on annual influenza (flu) vaccination for everyone six months and older in the U.S.
As of June 29, 2023, there are minor changes to the ACIP's flu shot recommendations, including, but not limited to, an acknowledgment of the updated flu vaccine composition for the 2023-2024 flu season.
And a change in the recommendations for vaccination of people with egg allergies.
Flu vaccination has many benefits. It has been shown to reduce the risk of getting sick with the flu and also to reduce the risk of more serious flu outcomes that can result in hospitalization or even death, says the CDC.
Rochelle P. Walensky, M.D., M.P.H.'s adoption of the ACIP recommendations makes them official C.D.C. policy. Providers should begin vaccinating patients according to C.D.C.'s recommended timing, which has not changed for the 2023-2023 influenza season in the U.S.
The C.D.C. says September and October are the best times for most people to get vaccinated.
Furthermore, flu vaccination in July and August is not recommended for most people, but there are several considerations for specific groups.
While influenza viruses are detected year-round, the exact timing and duration of flu seasons vary by country, says the World Health Organization (WHO). What happens in the Southern Hemisphere does not necessarily predict what will happen in the Northern Hemisphere, which includes the U.S.
The WHO recently published Influenza Update N° 448, which confirmed influenza detections remained low globally. Still, in the southern hemisphere, some countries reported variable changes in influenza detections in recent weeks, while detections in others seemed to have peaked as of June 26, 2023.
Additionally, Precision Vaccinations published an updated list of influenza vaccines and candidates conducting clinical trials.