Search API
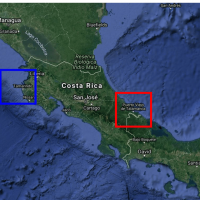
According to numerous reports, the Republic of Peru is confronted with another Guillain-Barré Syndrome (GBS) outbreak.
The Peruvian Government recently published Supreme Decree No. 019-2023-SA in the Official Gazette El Peruano, declaring a national health emergency due to the unusual increase in GBS cases in 18 of the country's 24 departments.
As reported on July 10, 2023, 182 cases of GBS have been confirmed, and four people have died since June 2023, per MercoPress.
Peru's president Dina Boluarte also issued a decree allocating $3.27 million for an action plan to improve patient care, including acquiring 5,000 immunoglobulin vials for treating patients affected by GBS.
Issued on July 8, 2023, this emergency declaration will be valid for 90 calendar days.
The U.S. Centers for Disease Control and Prevention (CDC) says GBS is the most common form of acute flaccid paralysis worldwide. It is characterized by motor weakness and other symptoms.
The CDC reported in a November 2020 Research Letter that from May 20–July 27, 2019, the Government identified 683 suspected or confirmed GBS cases in Peru.
Of the 683 GBS patients, 287 (42%) had descending muscle weakness, and 446 (65.3%) had ascending muscle weakness.
Of 426 patients for whom hospitalization data were available, 64 (15%) required mechanical ventilation.
Of 147 patients with an electrodiagnostic exam, 100 (68%) had acute motor axonal neuropathy.
The CDC stated this GBS outbreak was unusual because of the many cases. The incidence rate was nearly 25 times higher than expected.
And the rapid increase in numbers was followed by an equally precipitous decrease, which might suggest a point-source exposure.
Vaxart, Inc. recently announced positive topline data from the dose-ranging Phase 2 clinical trial of its oral pill bivalent norovirus vaccine candidate.
This study's preliminary results showed robust serum immune responses across all doses at Day 29 relative to Day 1.
Both doses showed a similar increase in serum antibody responses with no statistical difference between the medium and high dose arms, and the mucosal and cell-based assay data will be available later.
Dr. James F. Cummings, Vaxart's Chief Medical Officer, commented in a press release on July 6, 2023, "These data, additional forthcoming data from this study, and the data we expect from our norovirus challenge study, will help inform our selection of dosage levels in a larger Phase 2b study."
"And support an End-of-Phase 2 meeting with the U.S. Food and Drug Administration."
"Our bivalent vaccine is designed to target the most important genogroups, GI and GII, and specifically to cover the important strains, GI.1 and GII.4. GII.4 currently causes the majority of norovirus disease in humans."
This Phase 2 dose-ranging study demonstrated that the bivalent norovirus vaccine candidate was well tolerated, with a favorable safety profile.
This is the seventh clinical trial completed in Vaxart's norovirus program, and it supports previous findings of robust immunogenicity and benign tolerability.
As of July 10, 2023, Vaxart's vaccine is one of several norovirus vaccine candidates conducting clinical research.
Norovirus is a very contagious virus that causes vomiting and diarrhea. Anyone can get infected and sick with norovirus. The U.S. Centers for Disease Control and Prevention (CDC) says norovirus cases generally occur most frequently during late fall, winter, and early spring.
The CDC publishes the Norovirus Outbreak Map and posts Norovirus National Trends.

While official updates on H5N1-infected cats in Poland have increased over the past week, Polish authorities provided the European Centre for Disease Prevention and Control (ECDC) with an update, confirming that a total of 24 sick or dead cats were positive for influenza A(H5N1) virus (bird flu).
According to ECDC's testing guidance on avian influenza viruses in humans, any person exposed to sick/dead cats confirmed with A(H5N1) infection who develops symptoms should be tested as soon as possible for A(H5N1).
And persons exposed to sick/dead cats confirmed with A(H5N1) infection are advised to monitor their symptoms for 10–14 days after the last exposure and self-isolate if they develop symptoms.
They are also advised to wear a surgical mask or FFP2 respirator when in contact with others, seek medical advice and report it to public health authorities immediately.
And recently, the Italian Ministry of Health announced on July 6, 2023, that several dogs (and one cat) on a farm in Brescia, Italy, recently hit by avian influenza (bird flu), have seroconverted.
And the Italian Union of Public Medicine Veterinarians confirmed this HPAI H5N1 belonging to clade 2.3.4.4 b, and in particular to the H5N1-A/Herring_gull/France/22P015977/2022-like genotype, responsible for the cases reported in northern Italy in gulls.
This virus also has a mutation considered a marker of adaptation of mammalian viruses (T271A in the PB2 protein) with a possible increase in its zoonotic potential.
This mutation sparked considerable concern earlier this year when it was detected in infected mink in the fall of 2002, wrote the Avian Flu Diary.
The ECDC stated that considering the information and genomic data available until now and the fact that no human cases related to this event have been reported so far, ECDC assesses the current risk to the general public as low.
However, the risk is considered moderate for persons exposed to sick and/or dead cats confirmed with A(H5N1) infection, particularly if they belong to a vulnerable population group (immunocompromised people).
Considering the existing uncertainties, this assessment is preliminary and will be reviewed as soon as more information becomes available, says the ECDC.

The European Centre for Disease Prevention and Control recently published a Communicable Disease Threats Report (CDTR) for week #27, which included a mpox outbreak update.
The weekly number of mpox cases reported in the EU/EEA peaked in July 2022, and since then, a steadily declining trend has been observed.
Mpox is a viral disease, and the outbreak that began in May 2022 was driven by human-to-human transmission via close contact with infected individuals.
As of July 8, 2023, this CDTR confirmed since the last monthly update, 13 cases of mpox have been reported by Portugal (12) and Norway (1).
Portugal reported in the latest epidemiological update (June 30, 2023) that following three months with no new mpox cases, information is available for seven of the 12 patients; all were male, five (71%) were 20–29 years old, five presented with exanthema, and four are HIV-positive.
Based on evidence from the current outbreak and the declining number of new infections in the WHO European Region, the CDTR says the overall risk of mpox infection is moderate for men with sex with men and low for the broader population in the EU/EEA.
As of July 9, 2023, the leading mpox vaccine is JYNNEOS®.
As of June 27, 2023, 1,237,235 JYNNEOS doses (1st and 2nd) had been administered in 57 U.S. Jurisdictions. The U.S. CDC's vaccine advisory committee recently presented no recommendation for a third Jynneos dose.
Other sexually transmitted disease vaccine news is posted at Precision Vaccinations.

The Global Polio Eradication Initiative (GPEI) reported this week, three African nations reported continuing polio outbreaks.
Burkina Faso reported it's first circulating vaccine-derived poliovirus type 2 (cVDPV2) case of the year.
Chad reported two more cVDPV2 cases increasing its total for the year to ten.
And Nigeria reported six more polio cases, raising its total to 16 in 2023.
Furthermore, various countries reported cVDPV2-positive environmental samples as of July 5, 2023.
Previously, the World Health Organization (WHO) reconfirmed that the spread of poliovirus remained a Public Health Emergency of International Concern. As of July 2023, the WHO recommends travelers to polio-outbreak areas be fully vaccinated.
As of July 9, 2023, various polio vaccines are available worldwide.

As more countries reported measles outbreaks this year, the U.S. Centers for Disease Control and Prevention (CDC) is arming travelers with information on preventing this highly contagious, vaccine-preventable disease.
On June 29, 2023, the CDC updated its Travel Health Advisory that confirmed many international destinations are reporting increased numbers of cases of measles in 2023.
For example, Austria reported 130 cases of measles in 2023. Styria is the most affected region with 102 cases reported since the beginning of the outbreak in week 4, 2023.
And in Germany, 54 suspected and confirmed cases were reported as of July 2, 2023.
The CDC's Level 1 Global Measles advisory says travelers are at risk of measles if they have not been fully vaccinated two weeks prior to departure or have not had measles in the past and travel internationally to areas where measles is spreading.
And all international travelers, including infants 6–11 months of age and preschool-aged children, should be fully vaccinated against measles according to CDC's measles recommendations for international travel.
If you are not sure if you or your travel companions are fully protected against measles, schedule an appointment to see your clinician at least 1 month before traveling.
But, some people should not get a measles-containing vaccine. If you don’t think you can safely receive a measles-containing vaccine, talk to your clinician and consider making alternative travel plans.
Furthermore, international travelers should seek medical care if they develop a rash, high fever, cough, runny nose, or red, watery eyes. Travelers with suspected measles should notify the healthcare facility before visiting so staff can implement precautions to prevent the spread within the facility.
As of July 8, 2023, various measles prevention vaccines are available in the U.S. and worldwide.

The Republic of South Africa's National Institute for Communicable Diseases (NICD) today reported the year-long measles outbreak continues in the northern provinces.
As of July 7, 2023, the NICD confirmed in the past week (week #25) that ten laboratory-confirmed measles cases were detected across the country, most of which were from Limpopo (6).
In week #25, Limpopo reported a total of 5 cases.
The vaccination campaign in Limpopo province targeting the 5-15 years age group has come to an end with overall vaccination coverage of 56%.
To achieve a higher coverage rate, the NICD is informed that a mop-up campaign will be conducted through the end of August 2023.
Various measles vaccines are available globally, and most health agencies recommend full vaccination before visiting measles outbreak areas.
The NICD says measles is a highly contagious disease caused by an airborne virus. Complications are more serious in those who catch measles as young infants and in children who are malnourished.
Infected patients with measles present with fever and with a rash. The rash looks like small, red, flat spots over the body. The rash does not form blisters, nor is it itchy or painful.
Other signs include cough, red eyes, and a running nose.
Complications of measles can include diarrhea, dehydration, brain infection, blindness, and death.

Novavax Inc. announced yesterday that it had reached an agreement with Canada, under which the country would pay $349.6 million to settle the forfeiting of certain doses of the company’s protein-based COVID-19 vaccine.
Novavax COVID-19 vaccine brands include Nuvaxovid™, CovoVax, NVX-CoV2373, and TAK-019.
Since authorization, over 100 million doses of Nuvaxovid have been distributed globally in about 40 markets.
As reported by BNN on July 7, 2023, this development results from a significant decrease in global demand for COVID-19 vaccines, leading to a surplus of unused doses.
The World Health Organization weekly epidemiological update edition #150 confirmed that during the previous 28 days, the COVID-19 pandemic has declined since mid-2022.
In addition to the settlement, Novavax also entered into a revised contract with Canada’s public works and government services department.
The terms of the advance purchase contract were amended to reflect the reduced number of vaccine doses due for delivery and the revised schedule for the remaining doses.
On June 6, 2023, the U.S. Food and Drug Administration confirmed Novavax COVID-19 Vaccine, Adjuvanted, was available in the U.S. for certain people. And on July 6, 2023, Nuvaxovid received Full Marketing Authorization in Europe as a primary series in individuals aged 12 and older and booster in adults.