Search API
Gavi, the Vaccine Alliance, today announced support for human rabies vaccines for post-exposure prophylaxis (PEP) as part of routine immunization.
On June 13, 2024, Gavi stated eligible countries are receiving guidance on how to access these vaccines under Gavi’s cofinancing policy. The first round of applications will be accepted by mid-July 2024. Ninety-five percent of human rabies deaths occur in Africa and Asia.
“This commitment from Gavi is crucial and will expedite efforts to halt human fatalities caused by dog-mediated rabies,” said Dr Jérôme Salomon, Assistant Director-General for Universal Health Coverage, Communicable and Noncommunicable Diseases at WHO, in a press release.
“WHO will provide technical assistance to countries, not only to support their funding applications to Gavi but to draw up comprehensive plans of action that can deliver real progress towards the Zero by 30 goal.”
This development complements the ongoing global efforts of the Zero by 30 campaign, led by United Against Rabies partners, including the Food and Agriculture Organization, the World Health Organization, and the World Organisation for Animal Health, to eliminate dog-mediated human rabies by 2030.
In the United States, the Centers for Disease Control and Prevention reports bats, not dogs, are the leading source of rabies cases.

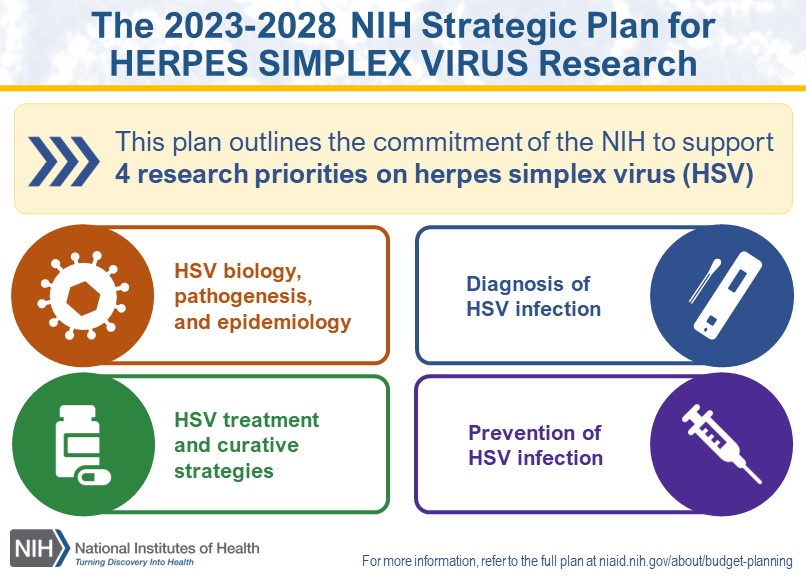
Researchers recently wrote that combining two monoclonal antibodies for treating chronic herpes simplex 2 (HSV-2) may provide a novel therapeutic option for this expanding disease.
The U.S. CDC says HSV constitutes a significant global health concern due to its wide range of clinical manifestations that can affect the skin and mucous membranes, the eyes, and the nervous system.
On May 28, 2024, the Journal of Biomedical Science published results from a study designed to develop a next-generation therapy by combining different antiviral monoclonal antibodies.
This research showed that the fully human antibody HDIT102 has the potential for further clinical development as a potent novel HSV therapeutic, particularly in combination with its clinical humanized ancestor antibody HDIT101.
HDIT101 is a humanized IgG that was previously investigated in phase 2 clinical trials.
Both antibodies induced the internalization of gB from the cell surface into acidic endosomes by binding distinct epitopes in domain I of gB and competing for binding.
CryoEM analyses revealed the ability to form heterogenic immune complexes consisting of two HDIT102 and one HDIT101 Fab bound to one gB trimeric molecule.
Both antibodies mediated antibody-dependent phagocytosis by antigen presenting cells which stimulated autologous T-cell activation.
In vivo, the combination of HDIT101 and HDIT102 demonstrated synergistic effects on survival and clinical outcome in immunocompetent BALB/cOlaHsd mice.
In conclusion, these researchers wrote, 'Antibody characteristics to inhibit cell-to-cell spread, to mediate uptake of cell-free viruses by phagocytic cells and concomitantly stimulate T-cell responses may promote cellular immunity and may have benefits in preventing recurrences.'
Antibody therapeutics are available to address a variety of diseases, and the U.S. Food and Drug Administration has approved more than 100 products.
Despite years of development, HSV vaccine candidates have yet to be approved. As of June 10, 2024, several herpes vaccines are conducting clinical research.
NeoImmuneTech, Inc. today announced that the European Medicines Agency (EMA) has granted Orphan Drug Designation (ODD) to NT-I7 for the treatment of Acute Radiation Syndrome (ARS).
This regulatory milestone follows the ODD granted to NT-I7 by the U.S. FDA for the same indication in November 2023.
ARS is a critical condition resulting from a high dose of radiation exposure, causing severe, sometimes fatal damage to the bone marrow and the immune system. No treatments are available that effectively promote T-cell recovery after such exposure.
NT-I7, a novel long-acting human interleukin-7 (IL-7), has shown promise to accelerate T cell reconstitution and enhance the immune response. It offers a potential solution for this unmet medical need. Clinical studies have shown that NT-I7 significantly boosts T cell counts while maintaining high safety and tolerability.
Luke Oh, Ph.D., President and Chief Executive Officer of NeoImmuneTech, said in a press release on June 4, 2024, "We are thrilled to see the deep interest of the scientific and regulatory community around the potential use of NT-I7 as a medical countermeasure in ARS."
"It is an important acknowledgment of the potential that NT-I7 holds in providing a beacon of hope for the treatment of ARS."
Examples of people who suffered from ARS are individuals exposed during the Hiroshima and Nagasaki atomic bombings and the Chernobyl Nuclear Power Plant incident firefighters.
NeoImmuneTech is actively developing NT-I7 in ARS through partnerships with the National Institute of Allergy and Infectious Diseases (NIAID) and Duke University. Recently, Duke University secured a $6 million grant from NIAID for NT-I7 development in ARS.

The U.S. Centers for Disease Control and Prevention (CDC) published a Morbidity and Mortality Weekly Report that confirmed Guillain-Barré syndrome (GBS) was identified as a potential safety concern following respiratory syncytial virus (RSV) vaccination.
On May 30, 2024, the CDC wrote that 'Findings are consistent with those from trials; reports of GBS (5.0 and 1.5 reports per million doses of Abrysvo and Arexvy vaccine administered, respectively) were more common than expected background rates.
Two deaths among RSV vaccine recipients who had been diagnosed with GBS were reported.
The CDC, the U.S. FDA, and the Centers for Medicare & Medicaid Services are conducting population-based surveillance assessments of RSV vaccine safety and risks for GBS. Findings from these studies will help guide the development of Advisory Committee on Immunization Practices recommendations.
About 10 million older adults have been vaccinated against RSV since August 2023.
As of June 3, 2024, three approved RSV vaccines for adults and one monoclonal antibody for infants are available in the U.S.

The Coalition for Epidemic Preparedness Innovations (CEPI) and Bavarian Nordic A/S today announced a partnership to advance the development of Bavarian Nordic's Modified Vaccinia Ankara (MVA-BN®) vaccine (JYNNEOS®, IMVAMUNE®, IMVANEX®) for children in Africa.
On May 30, 3034, CEPI stated it awarded $6.5 million to support a Phase 2 clinical study evaluating the immunogenicity and safety of MVA-BN in children from 2 to less than 12 years of age compared to adults for the prevention of smallpox, mpox, and related orthopoxvirus infections.
This phase 2 clinical trial will be conducted in one or more African countries and is planned to be initiated later in 2024. Notably, the study will also generate evidence on the vaccine in endemic African populations and could potentially support regulatory approval of MVA-BN in endemic countries.
The new trial follows the publication of a continental plan by Africa CDC and African Ministries of Health to strengthen mpox preparedness and response efforts, as well as the World Health Organization's framework for enhancing the prevention and control of mpox.
"We now understand that children suffer disproportionately from mpox, a concerning and neglected disease that has spread rapidly in recent years," said Dr. Richard Hatchett, CEO of CEPI, in a press release.
"To address the risk children face in DR Congo (Clade I) and other areas where the disease is endemic, CEPI is supporting this important trial which will provide key mpox vaccine safety and immunogenicity data in children."
Over 6,500 mpox cases and 345 deaths have been reported in DR Congo in 2024, with children accounting for the majority of infections and deaths. Mpox was initially identified in the DR Congo in 1970.
In 2024, Mpox cases were also confirmed in the Congo, Cameroon, Central African Republic, and Liberia.
The mpox strain behind the current outbreak in Africa, known as Clade I, is estimated to be fatal in around 8-12% of cases. In the U.S., Clade II has been detected since May 2022.
MVA-BN or JYNNEOS is a non-replicating smallpox-mpox vaccine approved in the U.S., and is available at certain clinics and pharmacies in 2024.

CSL Seqirus today announced it was selected by the U.S. Biomedical Advanced Research and Development Authority (BARDA) to complete the fill-and-finish process of the pre-pandemic vaccine for the U.S. government as part of the National Pre-Pandemic Influenza Vaccine Stockpile program.
Under the terms of the agreement revealed on May 30, 2024, CSL Seqirus will deliver approximately 4.8 million doses of a pre-pandemic vaccine well-matched to the H5 of the currently circulating H5N1 strain.
This acquisition of a pre-pandemic vaccine will increase BARDA's vaccine stockpile to support the U.S. government's pre-pandemic response. It is the fourth award CSL Seqirus has received from BARDA in response to sustained highly pathogenic avian influenza activity.
"The U.S. CDC maintains the risk to public health as low. We are closely monitoring the situation because we are acutely aware of the threat that influenza virus strains like H5N1 can pose and take seriously our role in preparedness efforts alongside our government and public health partners," said Marc Lacey, CSL Seqirus, Global Executive Director for Pandemic, in a press release.
"This agreement, building upon prior agreements with BARDA, will help support the U.S. government's ability to respond swiftly if the current avian flu situation changes."
On April 1, 2024, the U.S. Food and Drug Administration (FDA) Dr. Peter Marks informed the media that the U.S. stockpile of avian flu-specific vaccines would work well if deployed.
As of May 2024, FDA-approved avian influenza vaccines are not commercially available in the U.S. The FDA says annual flu shots are unlikely to protect people during avian influenza (bird flu, cow flu) outbreaks.
In Europe, the European Commission (EC) signed a framework contract on July 28, 2022, for the joint procurement of GSK's Adjupanrix, a pandemic influenza vaccine. That contact enables EC Member States to purchase up to 85 million vaccine doses during an influenza pandemic.
BARDA is part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services.
CSL Seqirus is a business of Australia-based CSL.




