Search API
The Houston Health Department recently reported a syphilis outbreak has significantly impacted women in eastern Texas and continues to expand in 2023.
As of July 13, 2023, there has been a 128% increase in syphilis cases among women and a nine-fold rise in congenital syphilis in Harris County.
In response to the syphilis outbreak, Houston's Health department is waving all clinical fees for sexually transmitted infections at its health centers. Untreated syphilis during pregnancy can result in a stillbirth or a baby's death soon after birth.
Syphilis testing is recommended in Texas at a woman's first prenatal visit, during the third trimester, and at delivery.
"It is crucial for pregnant women to seek prenatal care and syphilis testing to protect themselves from an infection that could result in the deaths of their babies," said Marlene McNeese Ward, deputy assistant director in the department's Bureau of HIV/STI and Viral Hepatitis Prevention, in a related press release.
"A pregnant woman needs to get tested for syphilis three times during pregnancy."
Statistics from the department indicate new syphilis infections rose from 1,845 in 2019 to 2,905 in 2022, a 57% increase.
Cases among women totaled 674 cases in 2022, up from 295 cases in 2019.
And congenital syphilis soared from 16 cases in 2016 to 151 cases in 2021.
Since reaching a historic low in 2001, the rate of syphilis has increased almost every year in the U.S., increasing 28.6% from 2020 to 2021.
Nationwide, men account for the most cases of syphilis.
In most cases, syphilis goes undetected because the signs and symptoms are misinterpreted or unnoticed.
If untreated, Treponema Pallidum, the bacterium that causes syphilis, remains in the body and begins to damage the internal organs, including the brain, nerves, eyes, heart, blood vessels, liver, bones, and joints, says the U.S. CDC.
As of July 15, 2023, the U.S. FDA has not approved a syphilis vaccine.
A systematic review and meta-analysis published today in Antimicrobial Resistance and Infection Control journal show that influenza vaccination is associated with significantly reduced antibiotic use.
The study focused on data from randomized controlled trials (RCT) and observational studies.
The RCTs showed that the effect of influenza vaccination on the number of antibiotic prescriptions or days of antibiotic use (Ratio of Means (RoM) 0.71, 95% CI 0.62–0.83) is stronger compared to the effect of pneumococcal vaccination (RoM 0.92, 95% CI 0.85–1.00).
These studies also confirm a reduction in the proportion of people receiving antibiotics after influenza vaccination (Risk Ratio (RR) 0.63, 95% CI 0.51–0.79).
And the effect of influenza vaccination in the European and American regions ranged from RoM 0.63 and 0.87 to RR 0.70 and 0.66, respectively.
However, the evidence from observational studies supports these findings but presents a less consistent picture.
Announced on July 14, 2023, this data supported the use of influenza vaccination as an important public health intervention to reduce antibiotic use and possibly control antimicrobial resistance.
In the northern hemisphere, the 2023-2024 flu season is forecasted to begin in the fall, with an ample supply of influenza vaccines available at most clinics and pharmacies in the U.S.
Gilead Sciences, Inc. today announced that the U.S. Food and Drug Administration (FDA) approved a supplemental new drug application (sNDA) for the use of Veklury® (remdesivir) in COVID-19 patients with severe renal impairment, including those on dialysis.
With this approval, Veklury is now the first and only approved antiviral COVID-19 treatment that can be used across all stages of renal disease.
This is essential news since more than 37 million people in the U.S. are estimated to have chronic kidney disease (CKD) and are at increased risk of COVID-19-related morbidity and mortality.
The FDA approval follows the European Commission's decision to extend the approved use of Veklury on June 26, 2023.
"Patients with advanced CKD and end-stage kidney disease (ESKD) are at high risk for severe COVID-19 with hospitalization and mortality rates remaining high, even for those who are vaccinated. With limited clinical trial information for COVID-19 patients with advanced CKD and ESKD, few antiviral treatment options currently exist for this population," said Meghan Sise, MD, Department of Nephrology at Massachusetts General Hospital, in a press release on July 14, 2023.
"This latest update to the prescribing information for remdesivir now includes patients with advanced CKD and ESKD, and this is an important advance for a population that remains highly vulnerable to the impacts of COVID-19."
The updated prescribing information for Veklury announced today does not require dose adjustments for renal-impaired patients and removes the requirement for eGFR testing before or during treatment with Veklury.
The leading cause of mosquito-borne disease in the continental United States is spread to people by the bite of an infected mosquito and has recently begun to spread in 2023.
Fortunately, most people infected with West Nile virus (WNV) do not feel sick, says the Centers for Disease Control and Prevention (CDC).
However, about 1 out of 150 infected people develop a serious, sometimes fatal, illness.
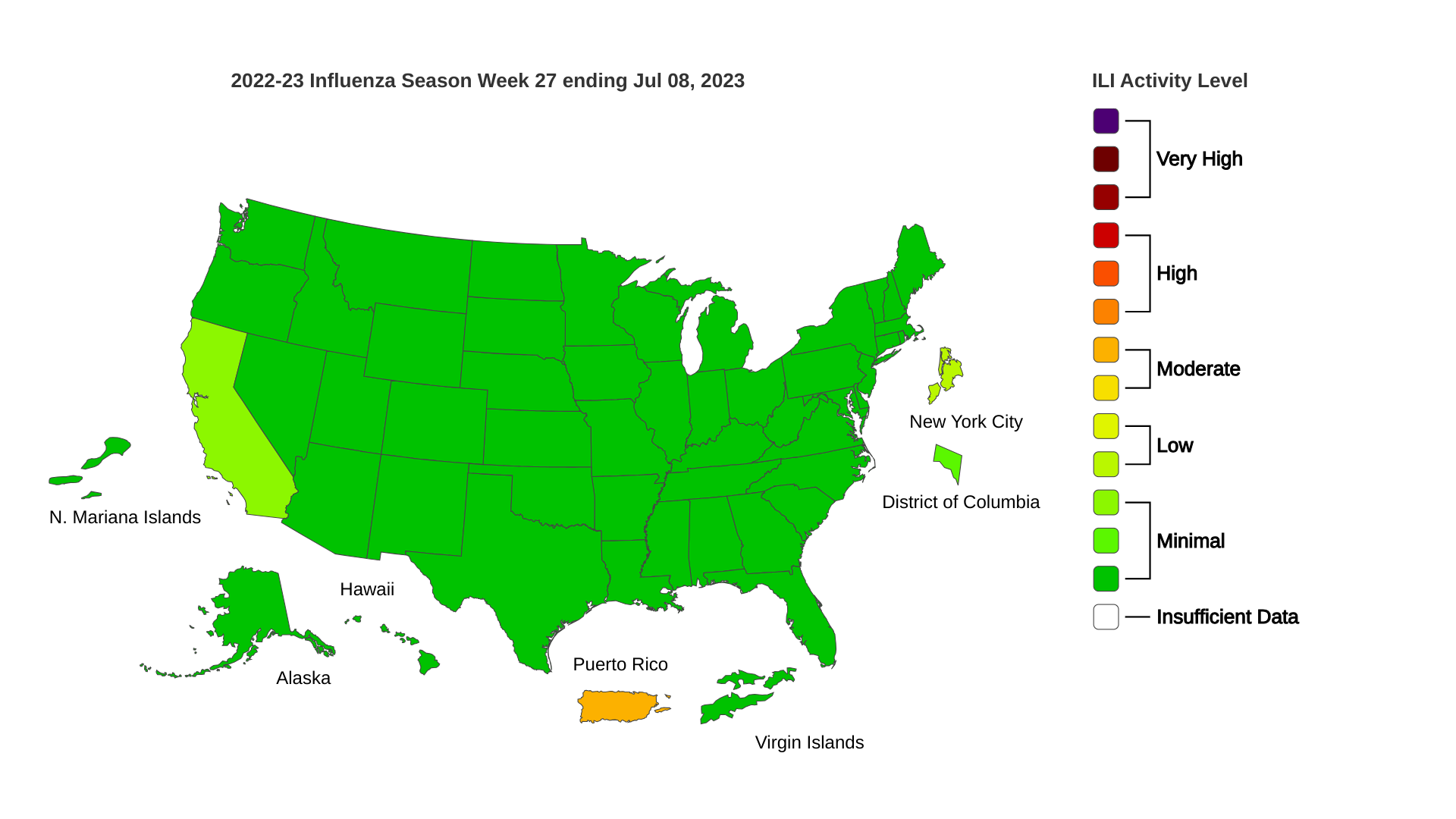
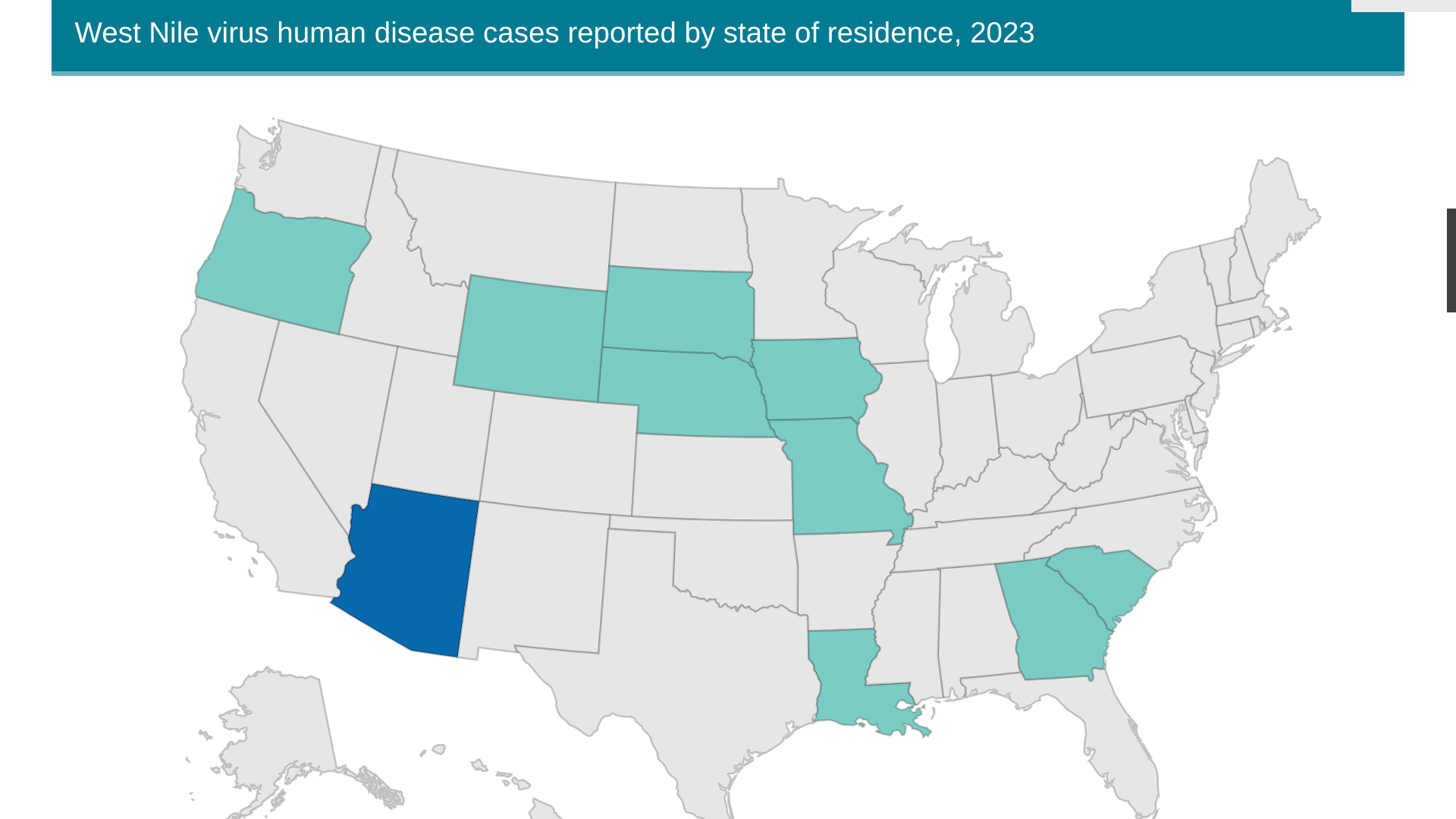
As of July 11, 2023, the CDC reported ten states have reported 36 human cases of WNV this year. So far, 23 neuroinvasive infections have been reported.
Not reported by the CDC was a WNV case confirmed in Dallas, Texas, during July 2023.
Arizona has the most WNV cases, with 25 reported in 2023. In 2021, Arizona reported over 1,700 WNV cases.
In 2022, there were 1,126 WNV cases, including 90 deaths in the U.S.
"It's important for people to be aware that there are many diseases transmitted by mosquitoes found in Texas," said Texas Department of State Health Services Commissioner Jennifer Shuford, MD, MPH, in a related press release.
"Most of these (WNV) diseases cause mild illness, but in rare instances, diseases like dengue or Zika can cause severe illness."
"We've even had a locally acquired malaria case in Texas this year, which underscores the importance of taking precautions to prevent mosquito bites."
There are no vaccines to prevent or medications to treat WNV in people as of July 14, 2023.
GSK plc today announced it has started shipping doses of its quadrivalent influenza vaccines to U.S. healthcare providers and pharmacies in preparation for the 2023-24 flu season.
GSK stated in a press release on July 13, 2023, that it expects to distribute over 40 million doses of its influenza vaccine to the U.S. market.
During the last flu season, over 170 million influenza vaccines were distributed in the U.S.
Both FLULAVAL QUADRIVALENT and FLUARIX QUADRIVALENT will be available in a 0.5mL, single-dose, pre-filled syringe, and are indicated for patients six months and older.
The U.S. CDC recommends an annual flu vaccination for anyone aged six months and older who does not have contraindications.
Regarding when to get a flu shot in 2023, the CDC suggests people speak with a doctor, nurse, or pharmacist about which influenza vaccine is best for their needs and co-administration options.

Genentech today announced that the Phase III OCARINA II clinical trial evaluating Ocrevus® (ocrelizumab) as a twice-a-year 10-minute subcutaneous injection met its primary and secondary endpoints in patients with relapsing forms of Multiple Sclerosis (MS) or primary progressive MS (RMS or PPMS).
In this clinical trial, Ocrevus subcutaneous injection was shown to be non-inferior to Ocrevus given by intravenous infusion (IV), as measured by pharmacokinetics (levels in the blood) over 12 weeks.
Additionally, Ocrevus subcutaneous injection was comparable with Ocrevus IV in controlling brain magnetic resonance imaging lesion activity over 12 weeks.
“These results give people living with MS the possibility to receive the transformational benefits of Ocrevus in the way best suited to their lives while freeing up time and healthcare resources,” said Levi Garraway, M.D., Ph.D., Genentech’s chief medical officer and head of Global Product Development, in a press release on July 13, 2023.
“This new subcutaneous injection will allow Ocrevus to be administered in 10 minutes twice a year, helping people living with MS to spend less time in treatment for this disease.’’
Ocrevus is not a vaccine but is a humanized monoclonal antibody designed to target CD20-positive B cells, a specific type of immune cell thought to be a key contributor to nerve cell insulation and support and nerve cell damage.
The Ocrevus 10-minute injection is designed to be administered without the need for IV infrastructure. Hence, it can potentially expand the usage of Ocrevus in MS centers without IV infrastructure or those with IV capacity limitations.
It also retains the twice-yearly dosing regimen of Ocrevus IV that has shown high persistence and adherence since becoming a standard of care MS treatment.
This provides an additional delivery option so that the administration of Ocrevus can be matched to the individual needs of patients and healthcare professionals.
The investigational subcutaneous formulation combines Ocrevus with Halozyme Therapeutics’ Enhanze® drug delivery technology.
Ocrevus remains the first and only therapy approved for RMS and PPMS, and more than 300,000 people have been treated globally.

The U.S. Transportation Security Administration (TSA) recently disclosed its premium security service has slowed traveler screening.
TSA's new data indicated that in June 2023, 88% of TSA PreCheck® passengers waited less than 5 min.
Previously, TSA PreCheck was averaging over 92% of people passed through airport security quickly.
Furthermore, this performance decrease may not have been related to increased activity.
According to TSA's Passenger Volumes report for late June and July 2023, air travelers only equaled but did not exceed the pre-pandemic activity last seen in 2019.
TSA PreCheck® is currently available at more than 200 airports with 85+ participating airlines nationwide. 'We encourage you to check with your airline before each flight for the most up-to-date information', says the TSA.