Search API
Consumers have recently expressed a desire to take a more proactive approach to Alzheimer's disease (AD) screening and a willingness to explore earlier diagnoses.
To meet that new trend, Quest Diagnostics today announced the availability of the AD-Detect™ Test for AD, the first blood test available for consumer purchase that helps assess the potential risk of developing AD based on a brain protein that contributes to the condition.
Quest's AD-Detect is a screening test that uses plasma, the liquid component of blood, from a single blood draw to evaluate levels of amyloid beta proteins to help detect early signs associated with the risk of developing AD.
Amyloid beta proteins are known to accumulate and form plaques in the brain, which are linked to the progression of Alzheimer's disease. AD-Detect evaluates the ratio of two peptides of amyloid beta, Aβ42, and Aβ40, in plasma.
"We are seeing much attention on emerging therapies for Alzheimer's disease, but with new treatment options will come the need to make screening and diagnosis more widely available. Blood tests like AD-Detect hold incredible potential to make Alzheimer's disease risk assessment both accessible and convenient," said Michael K. Racke, M.D., Medical Director of Neurology, Quest Diagnostics, in a press release on July 31, 2023.
The new consumer-initiated test utilizes the same expertise and technology as Quest's clinical AD-Detect Amyloid Beta 42/40 Ratio test, an analytically validated blood test that aids in assessing the risk of AD, which the company launched for physician ordering in early 2022.
As of August 1, 2023, there are no Alzheimer's disease vaccines available in the U.S.

The U.S. Fish and Wildlife Service (FWS) Incident Command Team recently confirmed implementing conservation strategies to help California condors in light of the Highly Pathogenic Avian Influenza (HPAI) bird flu outbreak.
As of July 28, 2023, over twenty-one Condors have died related to HPAI infections this year.
In May 2023, the United States Department of Agriculture's Agricultural Research Service announced the emergency use of a HPAI vaccine candidate to prevent additional deaths of California Condors.
The California Condor Vaccination Trial continued will continue into September 2023.
Blood samples from 13 birds will be collected at 21 and 42-days following vaccination to evaluate the immune response from two different vaccination approaches.
The first sample will be collected on August 8.
From a recovery perspective, three condors were transferred to the release site in Arizona to reacclimate to their home. A release date will be determined based on their behavior and weather.
The fourth bird that survived also has immunity to HPAI and will be released later as he is currently re-growing molted flight feathers.
The California Condor Recovery Program continues to implement standard operations, and we are hopeful this will include the release of juveniles in 2023. However, due to the dynamic nature of HPAI outbreaks and logistics around potential future vaccinations, adjustments will be made accordingly, wrote the FWS.
The ongoing bird flu outbreak reached Europe, Asia, and Russia in 2023.
Furthermore, the U.S. government has already approved one bird flu vaccine (Audenz™) for people and invested in vaccine candidates should a pandemic occur.

The U.S. CDC Advisory Committee on Immunization Practices (ACIP) is meeting on August 3, 2023, regarding the proposed recommendation for Beyfortus™ (Nirsevimab-alip), the first approved extended half-life monoclonal antibody (mAB) offering passive immunization to prevent lower respiratory tract infections (LRTI) caused by the respiratory syncytial virus (RSV).
This ACIP meeting draft agenda, from 11:00 am – 3:30 pm EDT, includes presentations on Feasibility/implementation plans for monitoring the safety and effectiveness of this RSV prevention drug and second-season clinical considerations.
The webcast link for this open-to-the-public digital meeting is here.
John Farley, M.D., M.P.H., director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research, commented on July 17, 2023, “Today’s approval (Beyfortus) addresses the great need for products to help reduce the impact of RSV disease on children, families, and the health care system.”
The FDA previously approved the Synagis® (Palivizumab) RSV mAb in 1998.
RSV is a virus that causes acute respiratory infection in individuals of all age groups. While most infants and young children experience mild, cold-like symptoms, some infants, especially with their first infection. RSV is transmitted from person to person through close contact with someone who is infected.
In most parts of the U.S., RSV circulation is seasonal, typically starting in Florida during the fall and peaking in the winter.

60 Degrees Pharmaceuticals Inc. today announced that the Canadian Intellectual Property Office issued a patent on using novel tafenoquine regimens for malaria prevention in malaria-naive individuals, and it will remain valid until December 2, 2035.
The Company was issued a similar U.S. patent in 2019.
Tafenoquine is the active molecule in the Company's U.S. Food and Drug Administration-approved regimen for malaria prevention, ARAKODA®.
ARAKODA, an oral tablet containing 100 mg of tafenoquine base, is an anti-malarial indicated for malaria prevention in individuals 18 years and older.
As of July 31, 2023, travelers or individuals at risk of contracting malaria are prescribed 2 x 100 mg tablets once per day for three days (the loading phase) before travel, 2 x 100 mg tablets weekly for up to six months during travel, then 2 x 100 mg in the week following travel.
Travelers from, and residents of, Canada and the United States, are usually malaria naive because they have not previously contracted malaria and thus lack immunity to the disease.
During July 2023, malaria outbreaks have been reported in Florida and throughout Central America in countries such as Costa Rica. And numerous African countries have confirmed malaria outbreaks.
The bite of an infective female Anopheles mosquito spreads malaria. The disease can cause fever, chills, and flu-like illness. If it is not treated, it can cause severe complications and death, says the U.S. CDC.
Typically, about 2,000 malaria cases are diagnosed in the United States yearly.
In Africa, there are two malaria vaccines currently being administered.
And on April 26, 2023, the United States Patent and Trademark Office issued 60 Degrees a patent covering the use of tafenoquine as a treatment for COVID-19 disease.

GC Biopharma today announced that the U.S. Food and Drug Administration (FDA) accepted the Company's resubmission of the Biologics License Application for its GC5107B (Immune Globulin Intravenous) for patients with primary humoral immunodeficiency (PI).
GC5107B is a liquid solution containing 10% immunoglobulin G (100 mg/mL) for intravenous infusion, manufactured from pooled human plasma from U.S. donors.
The FDA's Prescription Drug User Fee Act target action date is January 13, 2024. If approved, GC Biopharma could provide more treatment options for patients with PI in the U.S. next year.
PI disease comprises a large, heterogeneous group of disorders resulting from inborn errors of immunity.
Patients with PI cannot mount an immune response to pathogens and can experience recurrent bacterial, viral, fungal, and protozoal infections as a result.
As of July 31, 2023, global estimates project that up to six million people may live with PI.
While the U.S. immunoglobulin market size is estimated at US$ 10.4 billion in 2022, there have been sporadic shortages, says GC Biopharma. The manufacturing process includes three steps to reduce the risk of virus transmission.
Note: Immune Globulin Intravenous products are not preventive vaccines.
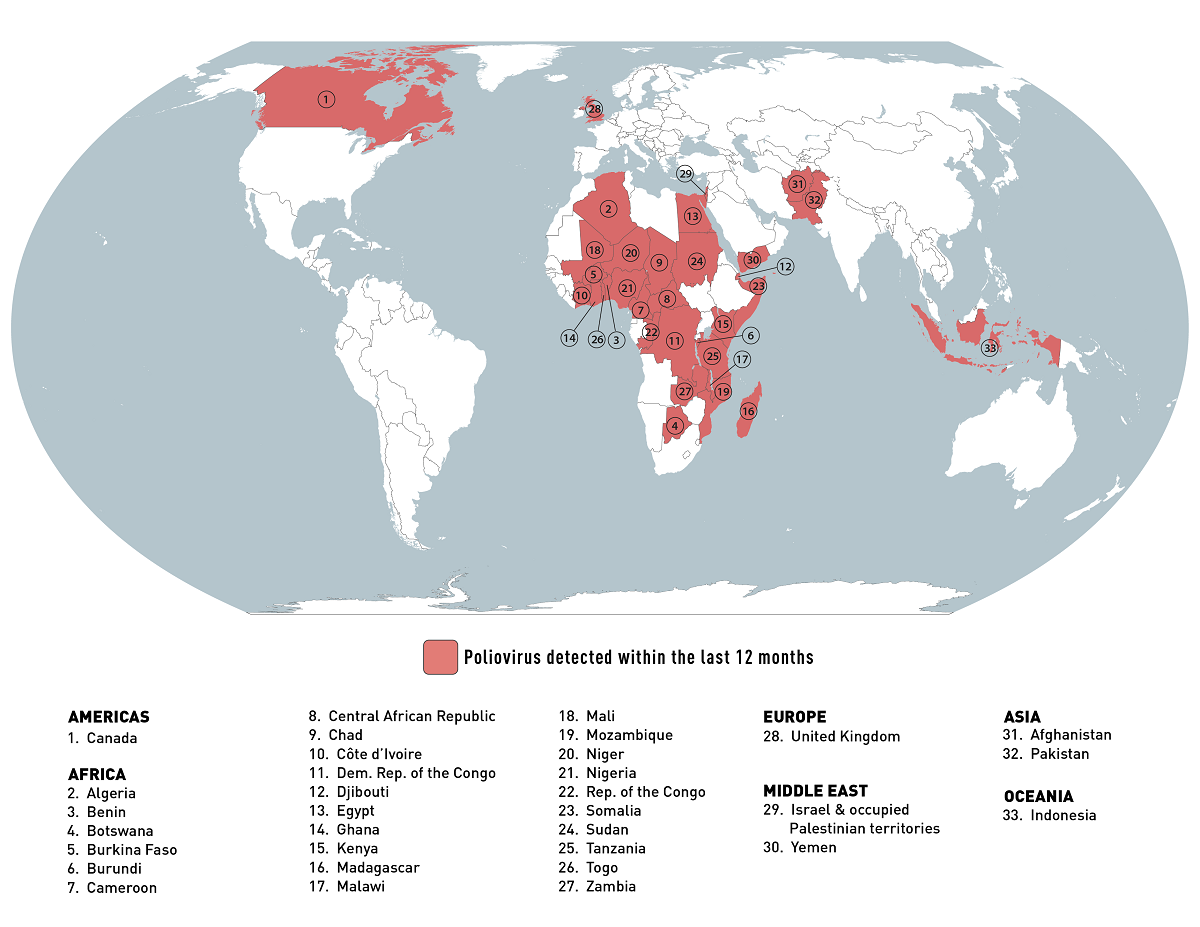
The World Health Organization (WHO) recently reported circulating vaccine-derived poliovirus type 2 (cVDPV2) cases in Africa.
The WHO confirmed on July 28, 2023, the United Republic of Tanzania reported the country's first cVDPV2 case and Kenya its first of 2023.
Tanzania's Ministry of Health notified the WHO the cVDPV2 virus was isolated from a case of acute flaccid paralysis (AFP) in the Rukwa region. Gene sequencing of the isolated virus has indicated close linkage with cVDPV2 currently circulating in South Kivu, Demographic Republic of the Congo.
According to the WHO-UNICEF estimates of national immunization coverage, the oral polio vaccine third dose (OPV3) and the inactivated polio vaccine first dose (IPV1) was 88% in Tanzania last year.
And on July 11, 2023, the WHO received an official report regarding detecting a cVDPV2 in two AFP cases and two asymptomatic healthy children community contacts in Kenya.
The genetic sequencing analyses showed that all four isolates are genetically linked to the cVDPV2 circulating in Banadir, Somalia.
Vaccine-derived poliovirus is a well-documented strain mutated from the strain originally contained in OPV.
OPV contains a live, weakened form of poliovirus that replicates in the intestine for a limited period, thereby developing immunity by building antibodies. On rare occasions, when replicating in the gastrointestinal tract, OPV strains genetically change and may spread in communities that are not fully vaccinated against polio, especially in areas with poor hygiene or overcrowding.
The lower the population's immunity, the longer this virus survives and the more genetic changes it undergoes.
In sporadic instances, the vaccine-derived virus can genetically change into a form that can cause paralysis, as does the wild poliovirus – this is what is known as a vaccine-derived poliovirus (VDPV).
The detection of VDPV in at least two different sources and at least two months apart that are genetically linked, showing evidence of transmission in the community is classified as cVDPV2.
In both countries, the WHO assesses the overall risk at the national level to be high due to the sub-optimal surveillance performance in some districts, sub-optimal vaccination coverage resulting in low population immunity, and the ongoing population movement across neighboring countries.
To alert international travelers, the U.S. CDC reissued its Level 2 - Practice Enhanced Precautions, Travel Health Advisory regarding the global polio outbreak on July 28, 2023.
The CDC says before any international travel, make sure you are up to date on your polio vaccines, and adults who previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of polio vaccine.
The nOPV2 vaccine is offered in Africa in July 2023. Approximately 670 million doses have been administered in more than 31 countries worldwide.
In the U.S., various polio vaccines are available at health clinics and community pharmacies.