Search API
As the threat of antibiotic resistance grows, researchers are developing ways to prevent recurrent and chronic urinary tract infections (UTIs) without using antibiotics, wrote Carissa Wong on May 2, 2024.
An article published in the journal Nature says the latest approaches include an oral spray vaccine.
In clinical trials, the pineapple-flavored Uromune™ (MV140) prevented recurrent UTIs in participants for up to nine years. The polyvalent bacterial whole-cell-based sublingual vaccine is sprayed under the tongue daily for three months.
Unfortunately, Uromune is currently unavailable in Canada or the United States. But it is offered in various countries.
Furthermore, scientists are also testing safer ways to treat UTI infections with antibiotics, which often cause side effects.
The anti-infective candidate RECCE® 327 (R327) was recently added to the World Health Organization's report on Antibacterial Agents in Clinical Development and Preclinical Development.
The U.S. CDC says UTIs are common infections caused by bacteria, often from the skin or rectum, entering the urethra and infecting the urinary tract.
UTIs are more common in females because their urethras are shorter and closer to the rectum. This makes it easier for bacteria to enter the urinary tract, says the CDC.
However, about 10% of men will also experience a UTI during their life.
Younger children may not be able to tell you about their UTI symptoms. While fever is the most common sign of a UTI in infants and toddlers, most children with fever do not have a UTI.
Access to the complete Nature article is at this link.

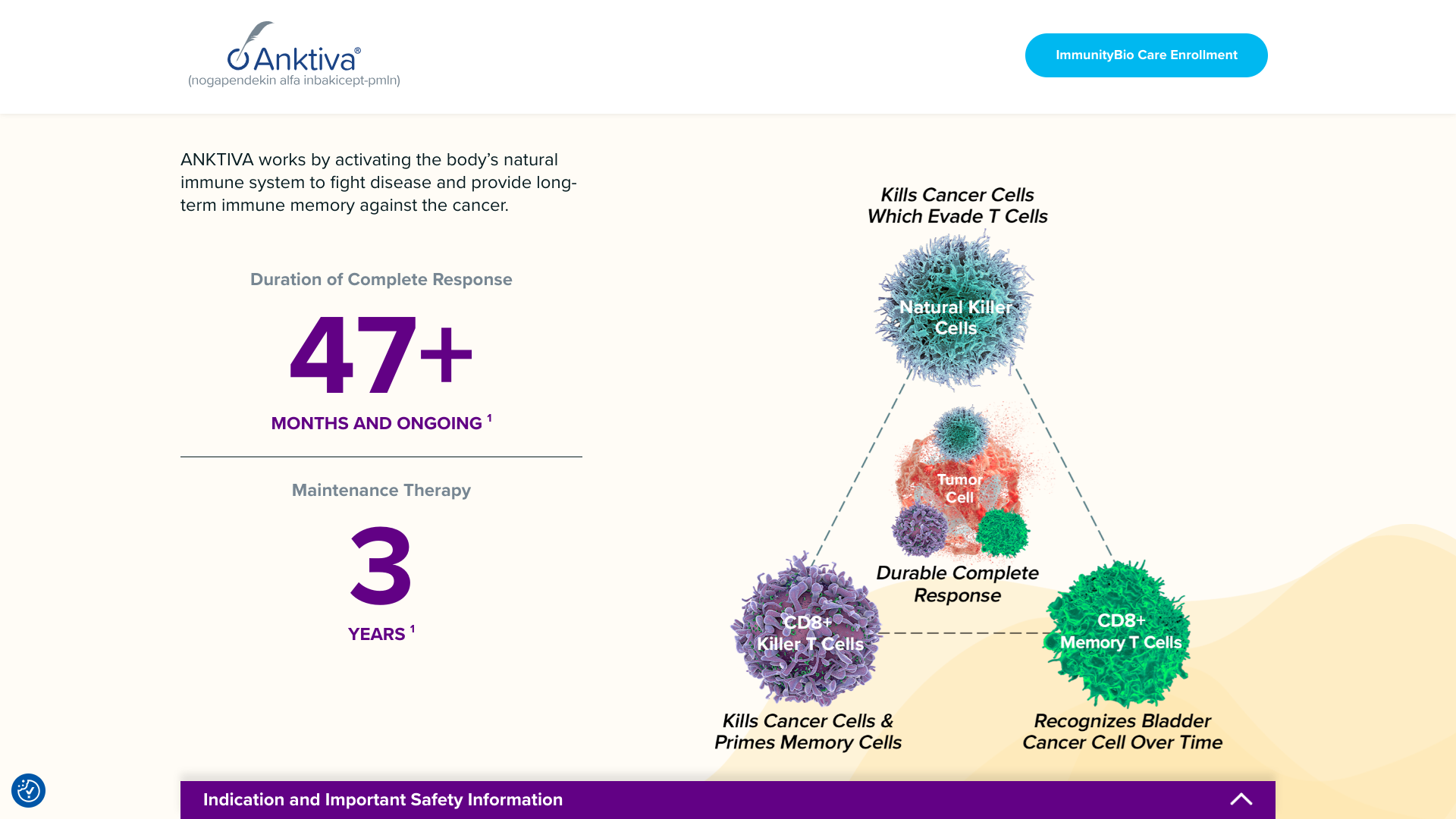
ImmunityBio, Inc. today announced the initial treatment of multiple patients in the U.S. to receive therapy with ANKTIVA®, the company's recently approved immunotherapy for Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC) carcinoma in situ.
The first bladder cancer patients to receive commercial doses are located throughout the U.S., and several are being treated by community urologists, as the therapy does not require any special handling or equipment that would limit its use to specialty medical centers.
ANKTIVA (nogapendekin alfa inbakicept-pmln) was approved by the U.S. Food and Drug Administration (FDA) on April 22, 2024, for the treatment of patients with BCG-unresponsive NMIBC CIS with or without papillary tumors.
The intravesical therapy employs a combination of ANKTIVA, an IL-15 agonist, in combination with the BCG vaccine.
The combination is the first FDA-approved immunotherapy in NMIBC that functions by activating the body’s NK and killer T-cell immune system to attack tumor cells while simultaneously activating memory T cells, leading to a prolonged duration of complete response exceeding 47 months for some patients.
“In addition to its unique mechanism of action, ANKTIVA can be readily administered by urologists in their own offices and clinics, enabling more patients to receive it in familiar settings from their own providers,” said Richard Adcock, President and CEO of ImmunityBio, in a press release on June 20, 2024.
“We look forward to ANKTIVA reaching more and more eligible NMIBC patients and for our science to deliver even more therapies from our pipeline.”
In May 2024, ImmunityBio announced it had drug substance sufficient for 170,000 doses of ANKTIVA for commercial and clinical trial use.
In the U.S., the American Cancer Society estimates there will be 83,190 new cases and 16,840 deaths from bladder cancer in 2024.
Recce Pharmaceuticals Ltd. today announced that its primary anti-infective candidate, RECCE® 327 (R327), was added to the World Health Organization's (WHO) report on Antibacterial Agents in Clinical Development and Preclinical Development.
The updated WHO report covers traditional and non-traditional antibacterial agents in development worldwide and evaluates to what extent the present pipeline addresses infections caused by priority pathogens.
R327 has been defined by the WHO as an ATP production disruptor and is the only compound under this category.
When targeted as the main mechanism of action, not secondary to other cell perturbation mechanisms, disruption of ATP production in bacterial cells has the potential to confer activity against both Gram-positive and Gram-negative pathogens.
Recce Pharmaceuticals CEO James Graham commented in a June 18, 2024, press release, "We are pleased that R327 has been included in the list of antibacterial products aimed at tackling the urgent global health threat posed by antibiotic resistance."
"There is a demand for new antibiotic therapies, and this report further showcases R327's potential as a novel treatment for a broad range of life-threatening and resistant bacteria."
Recce's anti-infective pipeline aims to address synergistic, unmet medical needs, such as urinary tract infections (UTIs).
UTIs are among the most common infectious diseases in the pediatric, female, and male populations.
The company anticipates releasing data in 2024 that is expected to pave the way for a Phase II UTI/Urosepsis efficacy trial, potentially establishing R327 as a frontline UTI treatment.

Clover Biopharmaceuticals, Ltd. today announced positive preliminary immunogenicity and safety data in the older adult cohort from its Phase 1 clinical trial evaluating SCB-1019, the company's bivalent RSV prefusion-stabilized F (PreF)-Trimer subunit vaccine candidate, which is based on Clover's Trimer-Tag vaccine technology platform.
Results indicate that SCB-1019 could potentially have a differentiated and favorable safety and reactogenicity profile compared to currently approved oil-in-water adjuvanted4 and/or mRNA5-based RSV vaccines.
"As the first RSV PreF vaccine candidate developed in China to enter the clinical trial stage and generate clinical data, SCB-1019 .... demonstrate broad and significant neutralizing antibody responses against both RSV-A and RSV-B as well as a favorable tolerability profile," said Joshua Liang, Chief Executive Officer & Board Director of Clover, in a press release on June 18, 2024.
"We look forward to the full clinical readout by the end of 2024 to support further development and strengthen our potentially differentiated profile for markets globally."
Preliminary results for RSV-neutralizing antibodies (nAbs) and safety for SCB-1019 at the selected dose level are summarized below:
Immunogenicity Results:
RSV-A nAbs: SCB-1019 induced geometric mean titers (GMTs) in RSV-A nAbs of up to 7,906 IU/mL compared to 1,078 IU/mL for placebo at Day 28.
RSV-B nAbs: SCB-1019 induced GMTs in RSV-B nAbs of up to 46,674 IU/mL compared to 12,185 IU/mL for placebo at Day 28.
Geometric Mean Fold Rise (GMFR): High baseline nAb titers at Day 0 (pre-vaccination), especially to RSV-B, were observed, potentially reflecting recent outbreaks near the clinical trial sites.
Thus, sub-analysis in subjects with the lowest quartile baseline nAb titers was performed: GMFRs for SCB-1019 were up to 8-fold for RSV-A nAbs and 11-fold for RSV-B nAbs at Day 28 compared to Day 0 (pre-vaccination). No increases in RSV-A or RSV-B nAbs were observed for the placebo on Day 28.
nAb results across both RSV-A and RSV-B appear to be in line or potentially favorable compared to other protein subunit RSV PreF vaccines.
Given that other monovalent RSV-A vaccines have previously observed lower immune responses and/or efficacy against RSV-B, we continue to support Clover's bivalent RSV-A/B approach, wrote the company.
Results further confirm that Clover's PreF antigens in SCB-1019 are in the stabilized prefusion and trimeric fo. This is additionally supported by exploratory immunogenicity results demonstrating significant increases in Site Ø and Site V nAb-competitive titers.
Safety & Reactogenicity Results: SCB-1019 was generally well-tolerated. Local and systemic adverse events (AEs) were generally mild and comparable to saline placebo. No serious adverse events, adverse events of special interest, or AEs leading to discontinuation were observed.
These preliminary results in older adults & elderly cohort (aged 60-85) are consistent with the positive results in younger adults (aged 18-59) announced earlier this year.
As of June 18, 2024, the U.S. FDA has approved three RSV vaccines and one monoclonal antibody (Beyfortus) for infants for the 2024-2025 RSV season.

Bavarian Nordic A/S today announced the completion of the rolling submission process with the U.S. Food and Drug Administration (FDA) for a Biologics License Application (BLA) for the licensure of its CHIKV VLP vaccine candidate for immunization against chikungunya virus infection in individuals 12 years of age and older.
CHIKV VLP is an adjuvanted VLP-based vaccine candidate for active immunization against chikungunya disease.
Initiated in April 2024, with acceptance from the FDA, the BLA could support a potential vaccine approval in the first half of 2025.
Bavarian Nordic also intends to submit a Marketing Authorisation Application (MAA) with the European Medicines Agency (EMA) by the end of the first half of 2024. The MAA has already been granted accelerated assessment, which means the CHIKV VLP vaccine could obtain approval from the European Commission in the first half of 2025.
“The completion of the BLA submission marks a significant milestone in the development of our CHIKV VLP vaccine and represents an important contribution to the development of preventative solutions for individuals 12 years of age and older at risk of chikungunya virus from bites by infected mosquitos. With the near-term anticipated MAA submission to EMA, we are looking towards potential approval of the vaccine in the first half of 2025 and subsequent launch in both the U.S. and E.U.,” said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release on June 17, 2024.
In the United States, the FDA has approved one chikungunya vaccine.
Chikungunya is a mosquito-borne viral disease that causes fever and severe joint pain. The World Health Organization (WHO) says the disease was first recognized in 1952. It is a ribonucleic acid virus belonging to the alphavirus genus of the family Togaviridae.
The WHO says chikungunya outbreaks have been identified in nearly 115 countries, primarily in the Region of the Americas. Most chikungunya cases in the contentital USA are travel related.
The U.S. Biomedical Advanced Research and Development Authority (BARDA) today announced up to $500 million in Project NextGen funding for multiple Phase 2b clinical trials to evaluate novel vaccines administered as a nasal spray or as a pill to protect against symptomatic COVID-19.
While currently approved COVID-19 vaccines are administered intramuscularly, they are limited in their capacity to induce a robust immune response in mucosal areas such as the mouth, nose, and gut, where the SARS-CoV-2 coronavirus first enters people.
Successful development of intranasal and oral vaccines would provide safe, effective, needle-free, easier-to-administer options with the potential to improve vaccine access.
“We learned a lot during the COVID-19 pandemic that we can use to better prepare for future public health crises. That includes finding new ways to administer vaccines to make it even easier for everyone to protect themselves from illness,” U.S. HHS Secretary Xavier Becerra said in a press release on June 13, 2024.
The project awards were made to:
Up to $453 million to Vaxart of San Francisco, California, developing an oral pill vaccine candidate, adenovirus serotype 5. BARDA will provide an initial $65.7 million for early trial milestones, with remaining funds provided as the effort successfully advances toward trial execution. Vaxart will execute its own Phase 2b clinical trials.
Approximately $34 million was donated to Castlevax, part of the Mount Sinai Health System in New York City, to develop an intranasal vaccine candidate, CVAX-01.
Approximately $40 million will go to Cyanvac of Athens, Georgia, to develop an intranasal vaccine candidate, CVXGA.
Castlevax and Cyanvac Phase 2b trials are in partnership with BARDA’s Clinical Studies Network.
These awards are just one component of BARDA’s Project NextGen medical countermeasures portfolio. To date, BARDA has leveraged more than $2 billion in Project NextGen funding to support the development of next-generation vaccines, treatments, and enabling technologies.
As of June 14, 2024, the U.S. government has approved three COVID-19 vaccines, while the World Health Organization has qualified 13 vaccines.

Sysmex Astrego AB announced today that it was awarded the Longitude Prize for antimicrobial resistance for developing a rapid antimicrobial susceptibility test for urinary tract infections (UTIs).
Sysmex Astrego received the $10.2 million award to incentivize the development of transformative point-of-care tests that will improve antibiotic treatment decisions.
Using a 400 microlitre urine sample on a smartphone-sized cartridge, the PA-100 AST System test can identify the presence of bacterial infections such as UTIs in just 15 minutes.
The goal is to replace the 2-3 day lab test process.
"Winning the Longitude Prize is the first true and biggest recognition that what we have been doing all these years was for a very important global cause," Ozden Baltekin, PhD, Sysmex Astrego director of program management, said in a press release on June 12, 2024.
Sysmex Astrego launched the PA-100 AST System in Europe in 2023 and intends to accelerate global expansion efforts.
UTIs are the most common bacterial infection, and around 50-60% of women develop one in their lifetime.
As of June 13, 2024, a UTI vaccine is available in certain countries, and new therapies are conducting late-stage development.