Search API
Each summer, influenza viruses are detected in both the northern and southern hemispheres. What happens in one hemisphere does not necessarily predict what will happen in the other because influenza viruses evolve and impact populations differently.
While the exact timing and duration of any flu season varies by country, the World Health Organization (WHO) recently reported most detections in late July 2023 are moderate.
The WHO recently published Influenza Update N° 450, indicating some countries in the southern hemisphere reported changes in influenza detections in recent weeks, while others seemed to have already peaked.
And Australia's Department of Health and Aged Care published report No. 8, which stated insufficient information to assess the 2023 influenza season's potential severity comprehensively.
The WHO and the U.S. CDC suggest international travelers speak with a healthcare provider about flu shot options before visiting countries reporting influenza outbreaks in August 2023.
As of August 5, 2023, most health clinics and community pharmacies in the U.S. offer various flu shots targeting 2023-2024 influenza viruses.

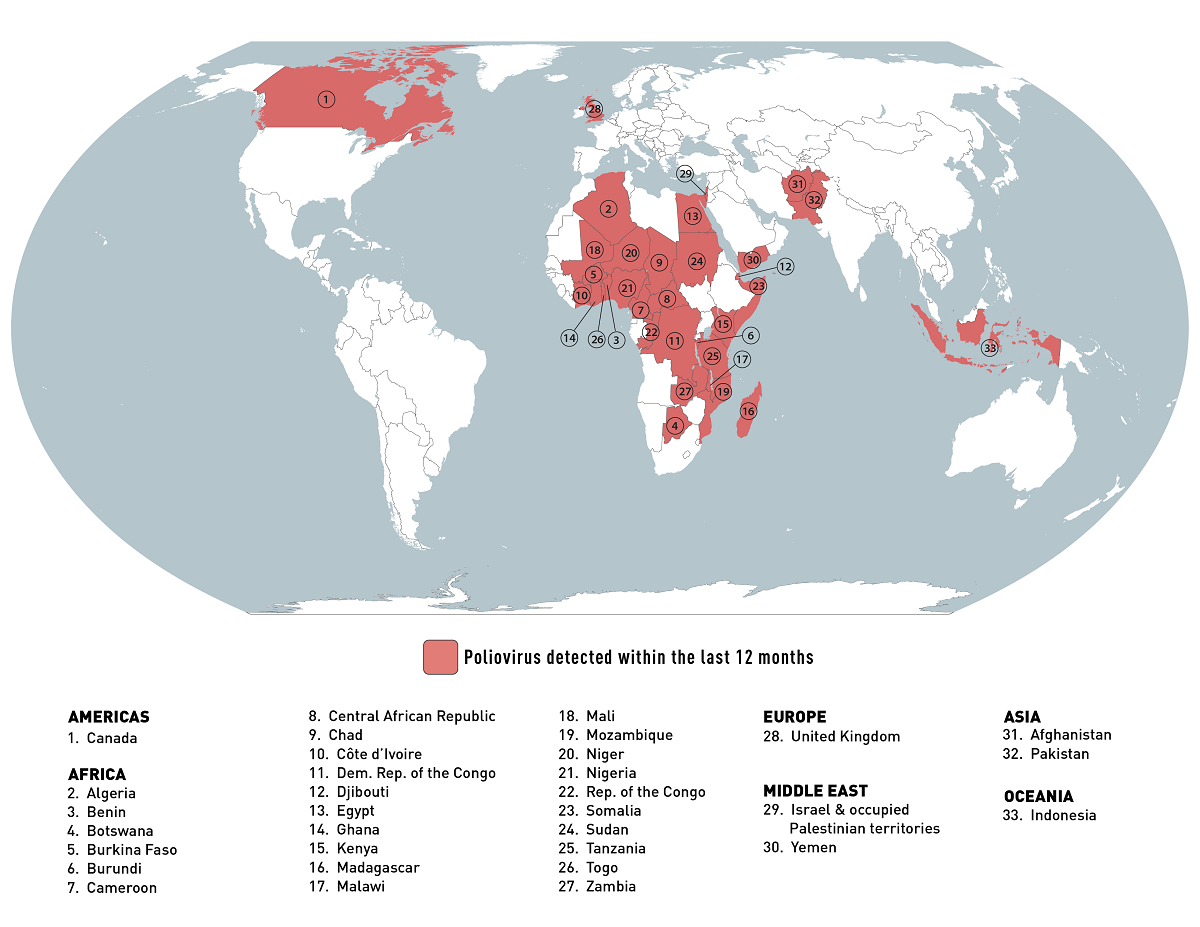
The Global Polio Eradication Initiative (GPEI) reported this week the Democratic Republic of the Congo (DRC) recently confirmed ten new cases of circulating vaccine-derived poliovirus type 1 (cVDPV1).
As of August 2, 2023, there are now 46 cases reported so far this year. There were 146 cases in 2022.
Additionally, the DRC reported four new cases involving circulating vaccine-derived poliovirus type 2 (cVDPV2).
There are now 61 cases so far this year and 367 cases reported in 2022.
In response to this polio outbreak, the U.S. CDC included the DRC in its Level 2 - Practice Enhanced Precautions, Global Polio Travel Health Notice.
The CDC said on July 28, 2023, adults who previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of an IPV polio vaccine before traveling to any of these 30 destinations.
In the U.S., IPV vaccinations are offered by travel pharmacies.
In the U.S., poliovirus is often detected in wastewater surveillance systems.
Separately, the GPEI published the 6th Transition Independent Monitoring Board report in July 2023, which evaluates the progress and challenges of transferring the responsibility of polio immunization and response efforts to national governments.
The report includes a series of recommendations to strengthen the process at the global, regional, and country levels.
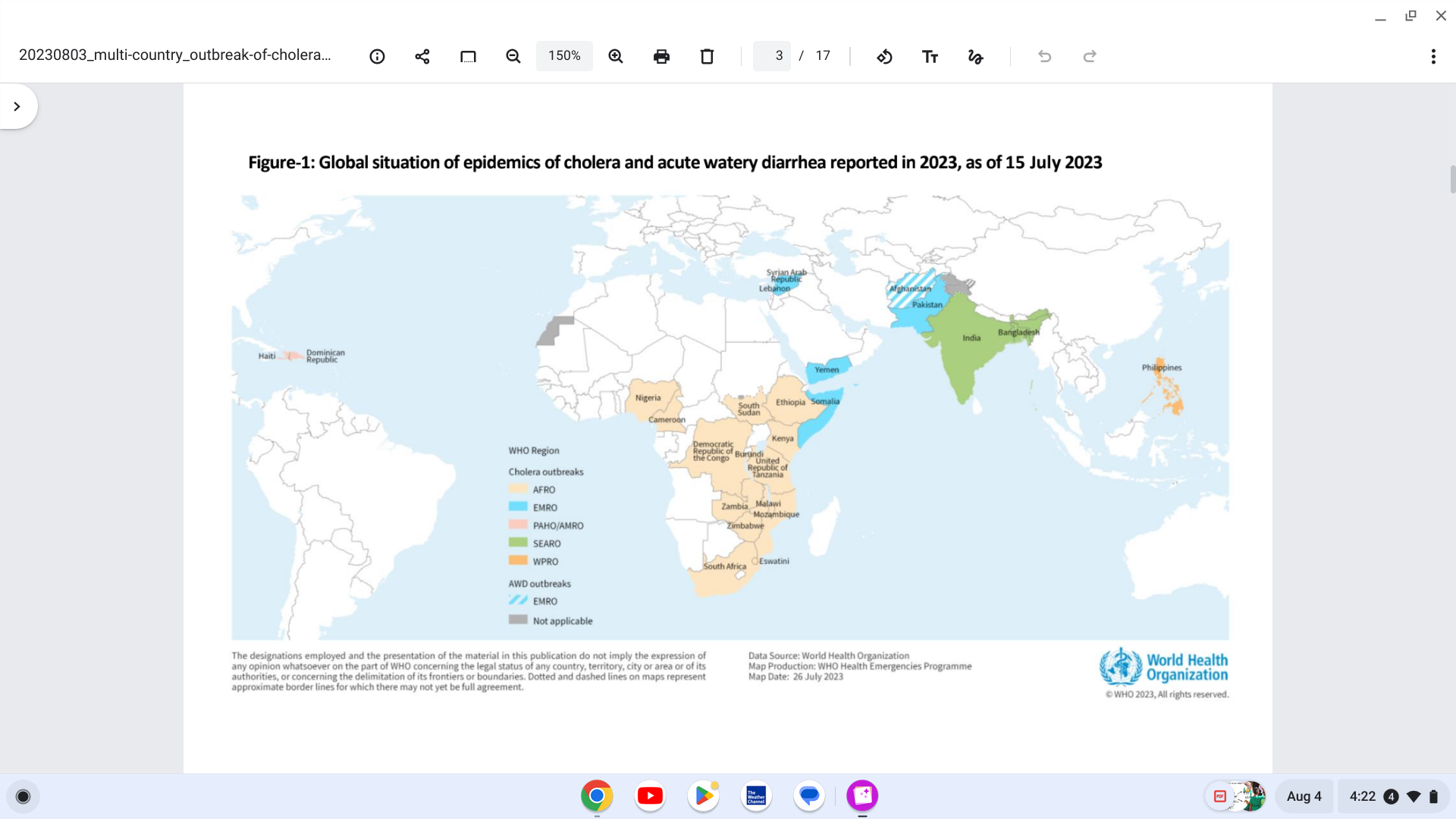
The World Health Organization's (WHO) latest multi-country cholera outbreak situation (#5) says that Based on the large number of outbreaks and their geographic expansion, WHO continues to assess the risk at the global level as very high.
As of August 4, 2023, twenty-five countries have reported cholera outbreaks since the beginning of 2023.
India became the 25th country on May 15, 2023.
The WHO African Region remains the most affected region, with 14 countries reporting cholera cases this year.
In the Region of the Americas, Haiti reported 54.826 cholera cases last year.
The overall capacity to respond to multiple and simultaneous outbreaks continues to be strained due to the global lack of resources, including shortages of the Oral Cholera Vaccine (OCV).
Since the start of 2023 and as of July 24, 2023, a total of 49.9 million OCV doses have been requested, of which 19.3 million (39%) have been approved for 11 countries. The available (not yet allocated) global OCV stockpile was 2.7 million doses.
In the current outbreak context, only one-dose courses have been validated and implemented in these reactive campaigns. Doses for preventive campaigns cannot be supplied due to the low global stockpile, says the WHO.
In the U.S., the CDC recommends that adults traveling to areas with active cholera transmission get vaccinated with a newly licensed cholera vaccine, Bavarian Nordic's Vaxchora®.
This OCV is indicated for active immunization against disease caused by Vibrio cholerae serogroup O1 in adults.
According to Vaxchora's manufacturers, the vaccine has limited availability in 2023.
Another vaccine. Valneva SE's Dukoral® is administered with a buffer solution that, for adults, requires 150 ml of clean water. Dukoral can be given to all individuals over the age of 2 years.
The U.S. Centers for Disease Control and Prevention (CDC) today reported the 166th influenza-associated pediatric death for the 2022-2023 flu season.
As of August 4, 2023, the CDC reported two additional influenza-associated pediatric deaths during week #30. Both deaths were associated with influenza A(H1N1) viruses.
Last flu season, there were only 47 flu-related pediatric deaths confirmed.
The CDC says the current flu shots offer protection against this type of influenza. For most persons, including children, who only one dose of an approved influenza vaccine is needed for the season.
Furthermore, vaccination should ideally be offered during September or October in the U.S.
And flu season in the Southern Hemisphere usually occurs between April and September.
Moreover, vaccination should continue throughout each flu season as long as influenza viruses are circulating.
Recent news indicates there will be plenty of flu shots available this season.
As of early August, about 100 million vaccines have already been distributed for the 2023-2024 flu season. Last season, the CDC reported that about 173.37 million flu shots were distributed in the U.S.
Various flu shots are available at health clinics and community pharmacies in the U.S.

The Access to Advanced Health Institute (AAHI) today announced that it has received an $18 million award from the National Institutes of Health (NIH) to develop a temperature stable, single-dose, RNA chikungunya vaccine candidate.
The NIH award disclosed on August 3, 2023, supports the development, preclinical testing, and human clinical evaluation of a vaccine that meets an increasingly urgent need for a reliable, abundant supply.
Chikungunya is a viral disease transmitted to humans through the bites of mosquitoes infected with the chikungunya virus (CHIKV), says the U.S. CDC.
Chikungunya outbreaks are significant causes of morbidity and mortality in Asia, Africa, and Latin America, for which no vaccine is currently approved.
From 2006–2021, 4,590 chikungunya cases in travelers were reported in the U.S.
Several vaccine candidates are conducting late-stage clinical trials, such as Valneva SE's VLA1553, a monovalent, single-dose, live-attenuated vaccine candidate.
AAHI's approach to an RNA vaccine against chikungunya differs from the RNA vaccines the U.S. FDA currently approves to prevent other diseases.
“This project will demonstrate the use of RNA vaccine technology to avoid some of the classic manufacturing challenges in the large-scale manufacture of live-attenuated vaccines,” said Emily Voigt, Ph.D., Principal Scientist, AAHI RNA Platform Lead, and Co-Principal Investigator for the award, in a press release.
Unlike other RNA vaccines, this candidate will generate a live "attenuated" virus that could induce strong and long-lasting immune protection against this mosquito-borne disease.
The new 5-year project builds upon work supported by the NIH (R43AI127053) for AAHI's proof-of-concept ground-laying work, which demonstrated that a liquid presentation of this live-attenuated chikungunya RNA vaccine candidate elicited strong immune responses in animals after a single dose, protecting them from mortality and joint swelling after being challenged with the virus (Voigt et al. 2021).
AAHI is a nonprofit biotech research institute located in Seattle, Washington, that combines the high-quality science of an academic research organization with the product development capabilities of a biotech company to help combat some of the world's deadliest diseases, including infectious diseases

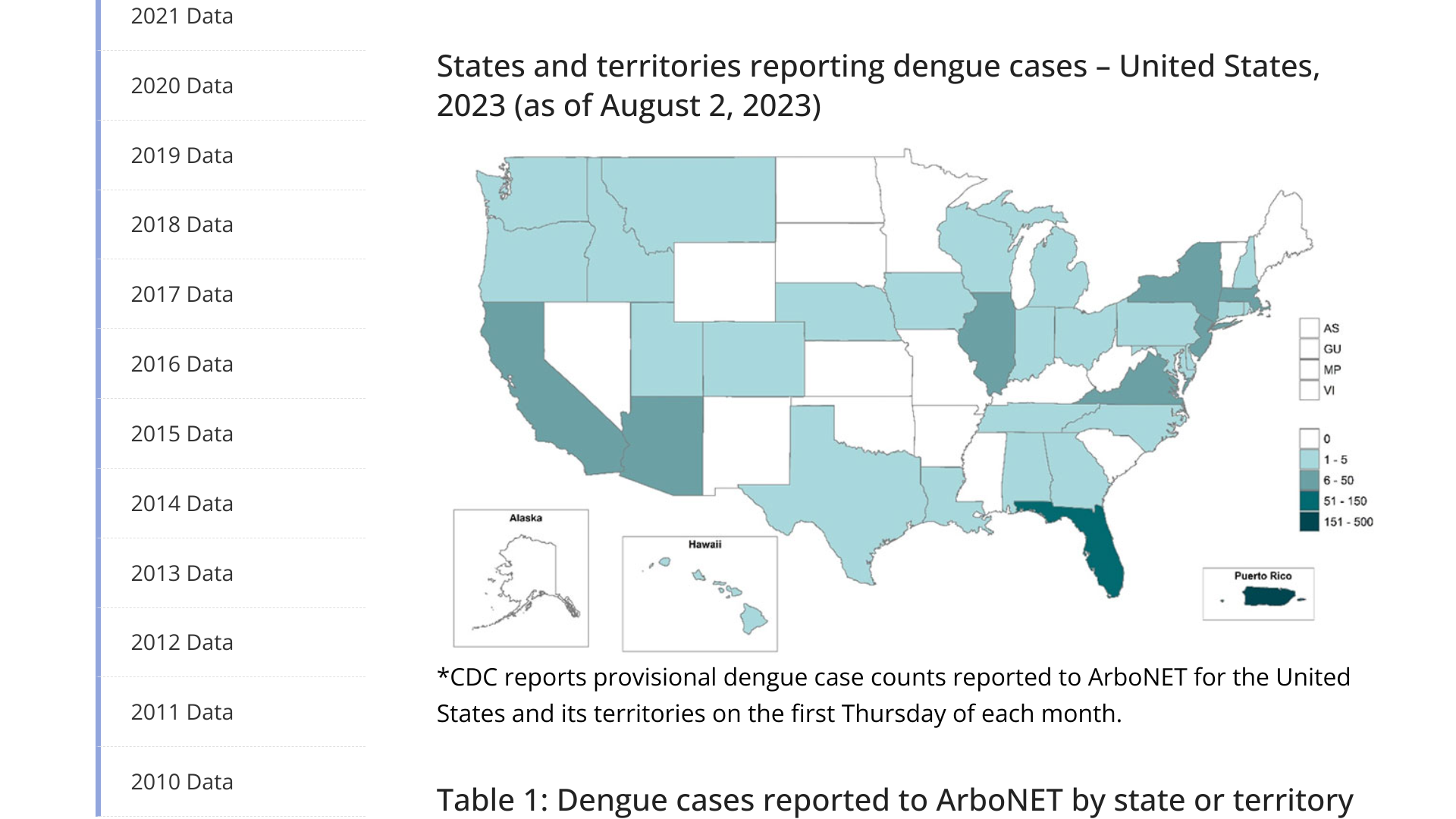
The U.S. Centers for Disease Control and Prevention (CDC) today reported the global dengue outbreak is impacting the United States.
On August 2, 2023, the CDC provisionally confirmed the U.S. States reported 225 dengue cases, and Territories reported 315.
For all of 2022, the CDC reported, the CDC reported 2,016 dengue cases.
From a state perspective, the Florida Health Department reported as of week #30 in 2023, there had been 147 travel-associated dengue cases. The majority (98) of travel cases were related to Cuba.
Florida has also reported six locally acquired dengue cases in 2023.
To alert travelers to their dengue health risk, the CDC recently issued Travel Health Notices for the Americas (2023), Africa/Middle East (July 21, 2023), Costa Rica, and Asia/Pacific Islands (July 25, 2023).
The CDC says dengue is a vaccine-preventable disease. As of August 3, 2023, two dengue vaccines are in use worldwide.
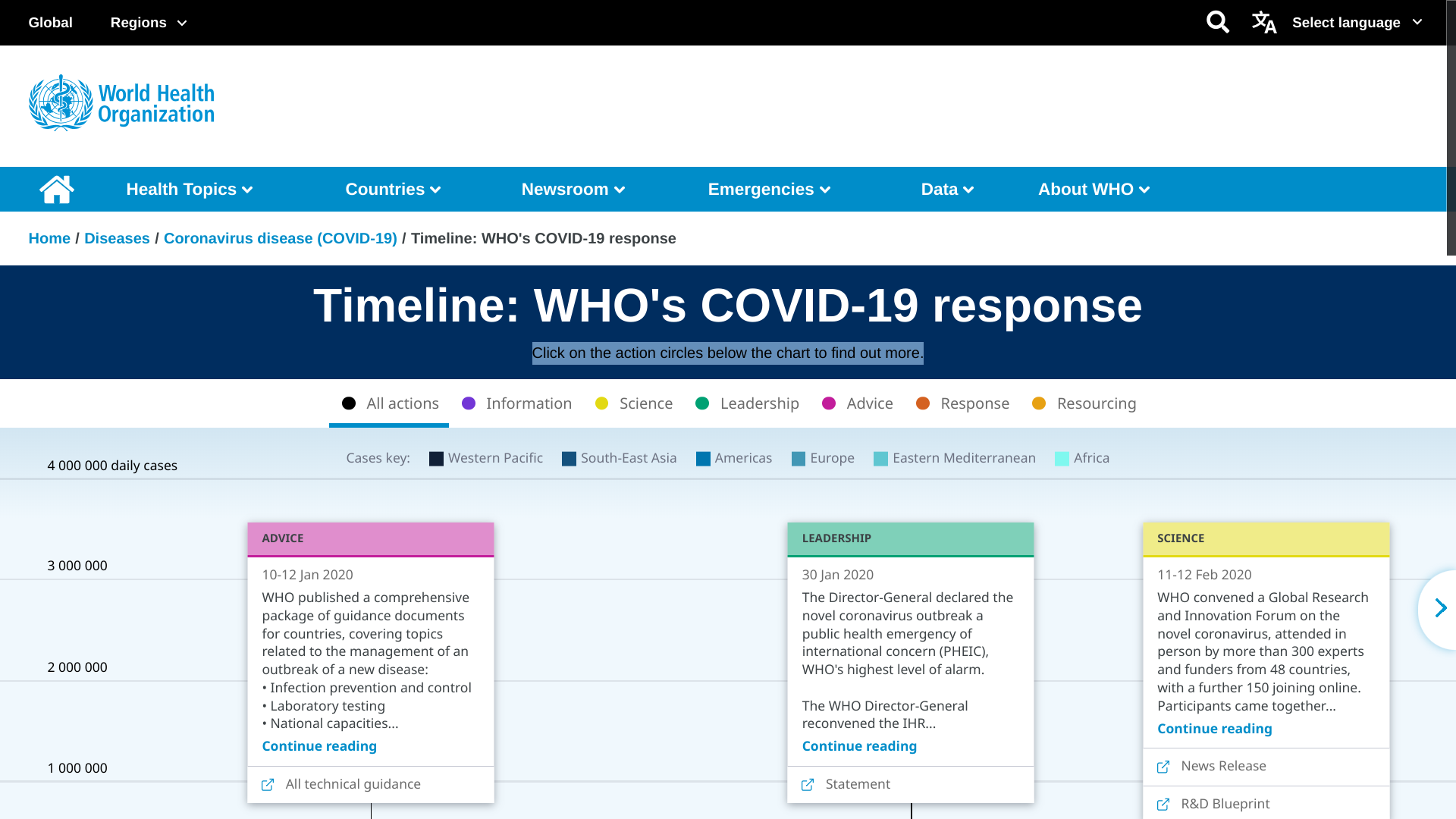
The World Health Organization (WHO) today reported (Edition 154) that during this recent 28-day period, 46% (107 of 234) of countries and territories reported at least one COVID-19 case, a proportion that has been declining since mid-2022.
While five WHO regions have reported decreases in both cases and deaths, the Western Pacific Region has reported an increase in patients and a decline in fatalities.
Globally, over 3,100 COVID-19-related deaths were reported between July 3 and 30, 2023.
Click on these action circles to learn more about the COVID-19 pandemic.
As of August 3, 2023, the WHO has LIsted 12 COVID-19 vaccines that are available in certain countries.
The U.S. CDC's Advisory Committee on Immunization Practices (ACIP) is meeting today to review Respiratory Syncytial Virus (RSV) Maternal/Pediatric vaccine and a long-acting monoclonal antibody.
On August 3, 2023, Dr. Grace Lee is leading the ACIP meeting agenda, which includes, but is not limited to, the following presentations:
-
Introduction - Dr. S Long
-
EtR summary for nirsevimab - Dr. J Jones
-
Nirsevimab implementation considerations - Dr. G Peacock
-
Clinical considerations for nirsevimab & Workgroup considerations / proposed recommendations - Dr. J Jones
At around 2 pm ET today, the ACIP is scheduled to vote on two recommendations.
Previously, the U.S. Food and Drug Administration approved Beyfortus (nirsevimab-alip) for the prevention of RSV lower respiratory tract disease in neonates and infants born during or entering their first RSV season and in children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
In the U.S., the RSV season generally starts in Florida in the fall. As of August 3, 2023, there have not been any RSV outbreaks reported this year.
The ACIP unanimously recommends routine use of Beyfortus™ to protect all infants below 8 months of age. The committee also voted unanimously to include Beyfortus in the Vaccines for Children program, supporting equitable access for all eligible infants.