Search API
CARsgen Therapeutics Holdings Limited today announced a collaboration agreement with Moderna Inc. to investigate CARsgen's investigational Claudin18.2 CAR T-cell product candidate (CT041) in combination with Moderna's investigational Claudin18.2 mRNA cancer vaccine.
CT041 (satricabtagene autoleucel) is CARsgen's autologous CAR T-cell product being investigated for potentially treating gastric, pancreatic, and other specified digestive system cancers.
It is currently in multiple ongoing clinical studies in China and North America.
"CT041 is the most advanced solid tumor CAR-T in development (pivotal phase II) and continues to show promise in treating gastric and pancreatic cancers. In our quest to make cancer curable, we are continuously exploring multiple modalities to eradicate tumors. Attacking tumors with CAR T-cell therapy in combination with a cancer vaccine could potentially provide greater clinical benefit to patients." said Dr. Zonghai Li, Founder, Chairman of the Board, Chief Executive Officer, and Chief Scientific Officer of CARsgen Therapeutics Holdings Limited, in a press release on August 21, 2023.
Dr. Li added, "Moderna has clearly established itself as a scientific and commercial leader in mRNA-based vaccines and therapeutics, and we are pleased to partner with Moderna to explore a potential synergism between our innovative therapies."
Moderna is developing an investigational off-the-shelf mRNA cancer vaccine that encodes for the Claudin18.2 protein, a tumor-associated antigen.
The collaboration contemplates conducting preclinical studies and a phase I clinical trial to evaluate CT041 in combination with Moderna's Claudin18.2 mRNA cancer vaccine.
"We are pleased to partner with CARsgen to explore the potential synergy of CAR-T with an investigational mRNA cancer vaccine that encodes for the Claudin18.2 protein. Claudin18.2 is a promising therapeutic target to potentially treat multiple cancer types with high unmet medical need. We continue to deliver on the promise of mRNA science to create a new generation of transformative medicines in oncology," added Dr. Lin Guey, Chief Scientific Officer of External Research Ventures, Moderna.
CARsgen is a biopharmaceutical company with operations in China, and the U.S. focused on innovative CAR T-cell therapies for treating hematologic malignancies and solid tumors.

A Perspective was recently published highlighting a seldom discussed and hard-to-spell mosquito-transmitted disease's impact on thousands of people's health in 2023.
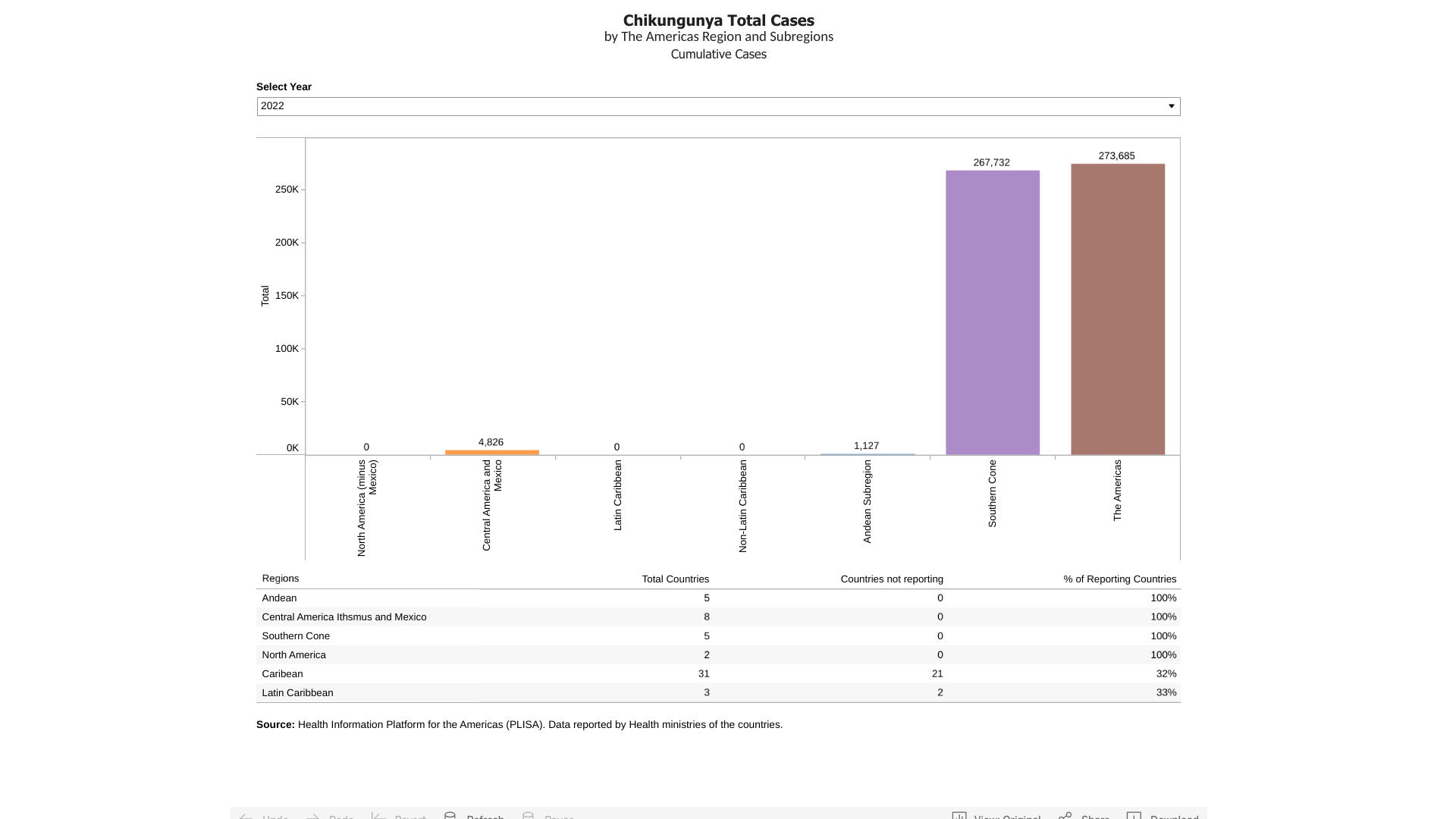
Chikungunya is an arboviral disease caused by the chikungunya virus (CHIKV), afflicting tropical and sub-tropical countries in 2023. Chikungunya outbreaks are primarily found in Africa, Asia, Brazil, and the Indian subcontinent.
For example, about 324,000 chikungunya cases have been reported in the Americas in 2023. As the Republic of Paraguay's ongoing Chikungunya outbreak confirms, this disease can be devastating.
Furthermore, it has been identified as a priority pathogen by the Coalition for Epidemics Preparedness Innovations (CEPI) and an emerging infectious disease.
And its impact has been measured.
Recent studies suggest that disability-adjusted life years (DALYs) due to CHIKV infection are as high as 106,089 DALYs lost globally.
The good news in 2023 is that significant progress has been made in developing several CHIKV vaccine candidates to prevent the disease.
Recently, Vaneva SE's VLA1553 monovalent, single-dose, live-attenuated vaccine candidate was assigned by the U.S. FDA a Prescription Drug User Fee Act review goal date at the end of 2023.
This perspective article published by the journal Nature on August 18, 2023, summarizes CEPI's efforts and strategic considerations for developing a CHIKV vaccine.
The Transportation Security Administration (TSA) recently announced that it was expanding TSA PreCheck® with Telos Corporation at ten airports in the U.S.
Telos began enrolling its first applicants during trial periods in Annapolis, Md., Chantilly, Va., Ashburn, Va., and Las Vegas.
Since TSA first launched the TSA PreCheck application program for low-risk travelers in December 2013, active membership in the program has grown to more than 15 million passengers.
About 99% of TSA PreCheck® passengers wait less than 10 minutes at 200 airports.
“TSA PreCheck is a trusted traveler program that improves overall aviation security and provides time and convenience benefits to its members,” said TSA Administrator David Pekoske in a press release on August 15, 2023.
“This expansion of enrollment providers will increase the network of locations where applicants may go to complete their TSA PreCheck membership.”
Moving forward, TSA PreCheck members may renew their memberships online with Telos or IDEMIA (TSA PreCheck’s original enrollment provider), regardless of who they enrolled with initially.
As of August 20, 2023, TSA reported airport screening activity had regained volumes last seen in 2019.

The U.S. Department of State issued an updated Level 1 travel advisory for the Argentine Republic on August 18, 2023.
The State Department says visitors should exercise standard precautions when visiting Argentina in 2023, as some areas have increased risk.
For example, the City of Rosario has been issued a Level 2: Exercise Increased Caution advisory.
The State Department says criminal elements are active in Rosario (Santa Fe province), increasing civil unrest.
Additionally, U.S. Embassy personnel are required to give advance notice before traveling to Rosario, which is located about 300 km northwest of Buenos Aires.
In 2022, Statista reported Argentine Patagonia received nearly 3.9 million overnight visitors, with January welcoming about 454 thousand people.
If you decide to travel to Argentina, enrolling in the free Smart Traveler Enrollment Program makes it easier to be located in an emergency.
From a health perspective, the U.S. CDC suggests various travel vaccinations, including for dengue outbreaks, when visiting Argentina in 2023.

Gavi today reported the Republic of Kenya is innovating to help every eligible child get four malaria vaccine doses in 2023.
As of August 18, 2023, about 96.5% of eligible children in Vihiga County in Kenya have received at least one RTS,S vaccine dose.
However, only 30% of eligible children in Vihiga are yet to complete the four-dose regimen that maximizes the WHO-approved malaria vaccine's effectiveness.
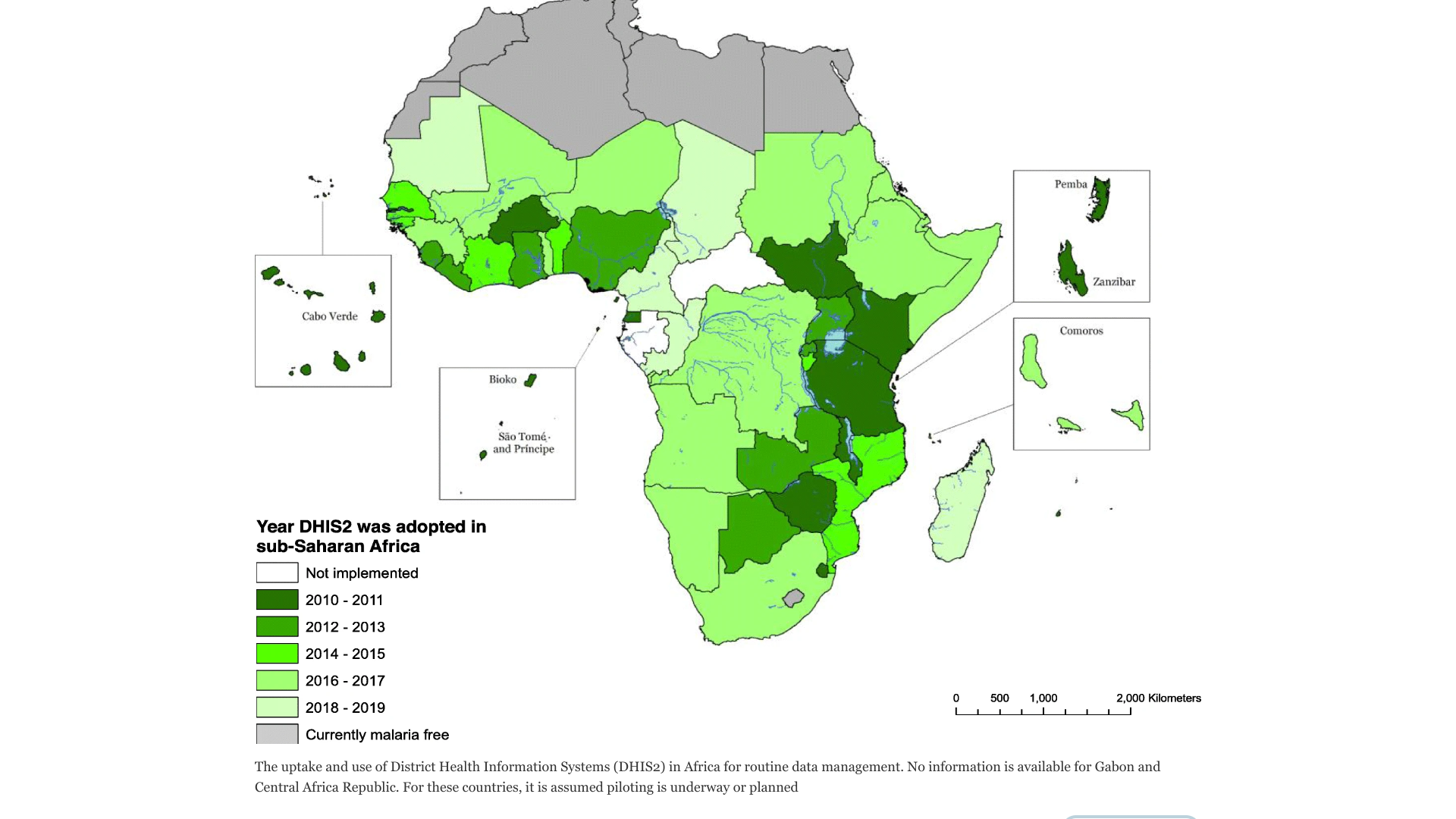
Part of the so-called "Lake-endemic region" in Kenya, a particularly mosquito-plagued set of districts abutting Lake Victoria, Vihiga has been offering malaria vaccinations since 2019.
The positive impact of malaria vaccination is already being measured.
More than a million Gavi-supported RTS,S (Mosquirix™) vaccine doses have been administered to some 400,000 children across the Lake-endemic counties, and Dr Abdourahmane Diallo, WHO Kenya Representative, confirms that those jabs, in concert with other malaria-control measures, have led to a demonstrable decline in case" .
The U.S. CDC Kenya"s Amy Herman-Roloff quantifies that decline at 50%.
The precise burden of malaria in sub-Saharan Africa has remained elusive, wrote researchers in an article published by the journal BMC Medicine.
Infection with Plasmodium falciparum is a frequent event for individuals living in stable transmission areas in Afric. Not all new infections cause illness in partdue tof acquired immunity, says the CDC.
In Africa, two malaria vaccines have been in use during 2023.
In addition to malaria, the CDC included Kenya in a recent polio outbreak travel advisory. When visiting polio outbreak areas, the CDC recommends travelers ensure they have been fully immunized.