Search API
The global search for innovative urinary tract infection (UTI) treatments recently received a $10 million boost.
Recce Pharmaceuticals Limited, a leading Australian developer of a new class of Synthetic Anti-Infectives, announced it had received binding commitments to raise about $10 million in new funds.
On July 2, 2024, Recce confirmed that the funds raised from the Placement will be used to advance clinical trials for intravenous use of R327 and topical applications of R327G, including Registrational Phase III clinical activities in Indonesia, Investigational New Drug (IND) enabling activities, working capital, and offer costs.
In a press release, Chief Executive Officer James Graham commented on the capital raising: "We are" delighted with the support of our capital raising from our existing shareholders and welcome new institutional shareholders to our register. We thank NorthStar Impact Fund for taking the time to understand Recce and the positive impact we aim to achieve."
Recce stated the Company will be fully funded through to FY2026 to fund significant IND-enabling clinical trials in Australia, covering intravenous and topical treatments for UTI/Urosepsis and ABSSSI, including Diabetic Foot Infections, as well as U.S. Department of Defence Burn Wound Program, Continued development of a pre-clinical portfolio, manufacturing expansion and provides the necessary capital to see Indonesian clinical trials for topical treatments through to commercialization.
On July 1, 2024, Recce clarified the Phase I/II clinical trial is an Open Label, Adaptive Design Evaluation, Crossover Study of the Safety, Pharmacokinetics and Pharmacodynamics of Various RECCE® 327 (R327) Intravenous Dose and Infusion Rates.
The primary trial outcomes were to evaluate the safety and tolerability of R327 administered at various infusion rates ranging from 15 to 45 minutes in healthy male and female participants, and to assess the plasma pharmacokinetics of R327 using the same infusion rates.
The secondary trial outcomes focused on evaluating the concentration of R327 in urine at various doses and infusion rates and examining the ex vivo pharmacodynamics, specifically the minimum inhibitory concentration, of urine and blood samples from participants. Trial outcomes were successfully achieved.
An independent data review has been conducted, and the positive safety and efficacy conclusions stated in the announcement released on June 28, 2024. A comprehensive data review will be conducted, with results to be made available to the Company, which are expected to align with findings to date.
In the United States, And in the United States, Pivya™ (pivmecillinam) recently gained the Food and Drug Administration (FDA) approval for treating adult women with uncomplicated UTIs. Pivya's availability in the U.S. is forecasted for 2025.

Voices of Alzheimer's, a national advocacy organization led by people living with Alzheimer's disease, today announced it celebrates the U.S. Food and Drug Administration's (FDA) decision to grant traditional approval of donanemab (Kisunla) for treating early Alzheimer's disease.
Following last year's first-ever traditional approval of a drug to slow the progression of Alzheimer's, today's decision builds on that progress by providing patients, care partners, and providers with another alternative to care during the early stages of the disease.
While Kisunla is not a cure, new treatment options still bring tremendous hope to affected families and offer priceless additional time for people in the early stages of Alzheimer's disease.
Jim Taylor, President & CEO of Voices of Alzheimer's and husband to Geri, who was diagnosed with Alzheimer's in 2012, said in a press release on July 2, 2024, "Today is a day for celebration in the Alzheimer's community. When doctors diagnosed my wife Geri with Alzheimer's, there was not a single approved treatment to slow the progression of the disease."
"Now, a decade later, we have two traditionally approved disease-modifying treatments and further advancements in the pipeline."
Taylor continued, "I am also encouraged by the evidence supporting stopping treatment with Kisunla when amyloid plaques are removed."
"People living with Alzheimer's and their care partners already face significant costs and burdens in their day-to-day lives. The possibility of stopping treatment could translate to lower costs and a reduced treatment burden."
In light of this news, Voices of Alzheimer's reiterates our call for the Centers for Medicare and Medicaid Services to remove coverage with evidence development requirements for the entire class of monoclonal antibody treatments for Alzheimer's.
The total cost of Kisunla will vary by patient based on when they complete treatment, says Eli Lilly.
Lilly Support Services for Kisunla is a free support program committed to helping patients navigate treatment with Kisunla. The program includes coverage determination assistance, care coordination, nurse navigator support, customized support, and resources. For more information, visit www.Kisunla.Lilly.com or call 1-800-LillyRx (1-800-545-5979).
Alzheimer's disease preventive vaccine candidates continue to be researched in clinical trials.

In the past two years, H5 influenza virus subtypes have caused severe disease in birds and mammals in the United States. Because of various media reports, there is concern about the risk of these viruses spreading to humans, which could generate another pandemic.
On June 27, 2024, the U.S. Centers for Disease Control and Prevention (CDC) confirmed that the risk to people is very low. However, the U.S. government continues its multi-year effort to prepare for this risk.
Moderna, Inc. today announced a project award of $176 million to accelerate the development of mRNA-based pandemic influenza vaccines. The award program is within the U.S. Department of Health and Human Services (HHS).
The project award will support the late-stage development of an mRNA-based vaccine to enable the licensure of a pre-pandemic vaccine against the H5 influenza virus.
This new HHS agreement also includes additional options to prepare and accelerate responses to future public health threats.
"mRNA vaccine technology offers advantages in efficacy, speed of development, and production scalability and reliability in addressing infectious disease outbreaks, as demonstrated during the COVID-19 pandemic," said Stéphane Bancel, Chief Executive Officer of Moderna, in a press release on July 2, 2024.
In July 2023, Moderna initiated a Phase 1/2 study to generate safety and immunogenicity data for the investigational pandemic influenza vaccine (mRNA-1018) in healthy adults. The study includes vaccine candidates against H5 and H7 avian influenza viruses.
Results from the study are expected in 2024 and will inform Phase 3 development plans.
Currently, there are U.S. FDA-approved pandemic vaccines (Audenz) and various development initiatives underway, funded by the U.S. government.
Furthermore, the CDC has confirmed annual flu shots may not be effective against these influenza subtypes.

PharmaJet® today announced that their Tropis® Intradermal (ID) Needle-free System will be used in a house-to-house polio immunization campaign.
Over a quarter million PharmaJet’s needle-free intradermal syringes have been provided to support this initiative.
The campaign will be conducted in two rounds to reduce the immunity gap significantly against type-2 poliovirus. Young children will receive the needle-free polio vaccine and novel oral polio vaccine (nOPV2) to achieve 95% coverage in each round.
The polio campaign, a collaboration of the African Field Epidemiology Network, WHO, UNICEF, BMGF, GAVI, and U.S. CDC, targets over 170,000 children in Somalia.
The most recent evidence for human circulating vaccine-derived polio virus-2 was in March 2024.
Through the Somalia Emergency Action Plan, the country will continue to work with humanitarian partners to reach about 1.5 million zero-dose children, most of whom live in the country’s highly populated central and southern areas.
Paul LaBarre, Vice President of Global Business Development at PharmaJet, commented in a press release on June 27, 2024, “In Somalia, we are eager to build on previous house-to-house campaign experience that demonstrates how needle-free enables vaccination teams to move quickly and achieve high coverage without the burden of sharps waste management and with reduced vaccine volume and cold chain logistics.”
The U.S. CDC reissued a Global Polio Alert on May 23, 2024, regarding polio outbreaks and poliovirus detections in 34 countries. The CDC recommends that visitors to these countries be fully vaccinated against polio.

Dynavax Technologies Corporation today announced that the first participant has been dosed in a Phase 1/2 clinical trial evaluating the safety, tolerability, and immunogenicity of Z-1018, the company's investigational vaccine candidate being developed for the prevention of shingles (herpes zoster).
The Phase 1/2 randomized, active-controlled, dose escalation, multicenter trial is expected to enroll approximately 440 healthy adults aged 50 to 69 years at trial sites in Australia and will evaluate the safety, tolerability, and immunogenicity of Z-1018 compared to the Shingrix® vaccine.
Key objectives of the trial include selecting the optimal glycoprotein E (gE) protein dose level and dosing schedule for further clinical development. The Phase 1/2 trial will also support the validation of a Patient-Reported Outcome measurement tool to differentiate Z-1018 on tolerability and support potential label claims.
"We believe there is an opportunity to develop an improved shingles vaccine with a significantly better tolerability profile than the market-leading shingles vaccine. One of the unique advantages of our vaccine candidate is CpG 1018 adjuvant's established safety and tolerability profile, combined with its ability to induce strong CD4+ T-cell responses, which are thought to be critical in preventing the reactivation of the herpes zoster virus," said Rob Janssen, M.D., Chief Medical Officer of Dynavax, in a press release on June 27, 2024.
Dynavax anticipates reporting top-line immunogenicity and safety data in the second half of 2025, including comparing CD4+ T-cells one month after the second of two vaccine doses.
According to the U.S. CDC, shingles risk increases with age and in people with weakened immune systems. About 33% of people in the United States develop shingles at least once, and fewer than 100 people die of shingles each year.
As of June 2024, there are four approved shingles vaccines and several vaccine candidates conducting clinical research.

Bavarian Nordic A/S today announced the submission of a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) seeking approval of the Company’s vaccine candidate for immunization to prevent disease caused by chikungunya virus infection in individuals 12 years and older.
CHIKV VLP is an adjuvanted VLP-based, single-dose vaccine candidate for active immunization to prevent disease caused by CHIKV infection.
The MAA application was granted accelerated assessment by the Committee for Medicinal Products for Human Use in February 2024, supporting the potential approval of the vaccine by the European Commission in the first half of 2025.
In the past 20 years, the chikungunya virus has emerged in several previously non-endemic regions in Asia, Africa, southern Europe, and the Americas, often causing large, unpredictable outbreaks.
The ECDC says Chikungunya is not endemic in mainland Europe, and most cases are travelers infected outside of the mainland European Union/European Economic Area.
“Our CHIKV VLP vaccine is designed for ease of use in individuals 12 years of age and older at risk of chikungunya virus and represents an important contribution to the development of preventative solutions against this debilitating disease,” said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release on June 26, 2024.
The MAA submission includes results from two phase 3 clinical trials in more than 3,600 healthy individuals 12 years and older. The results showed that the CHIKV VLP vaccine was highly immunogenic, as demonstrated by the strong induction of Chikungunya neutralizing antibodies 21 days after vaccination, with antibody titers equal to or above the threshold agreed with authorities as a marker of seroprotection in the majority of individuals. The CHIKV VLP vaccine was well-tolerated across both studies, and vaccine-related adverse events were mainly mild or moderate in nature.
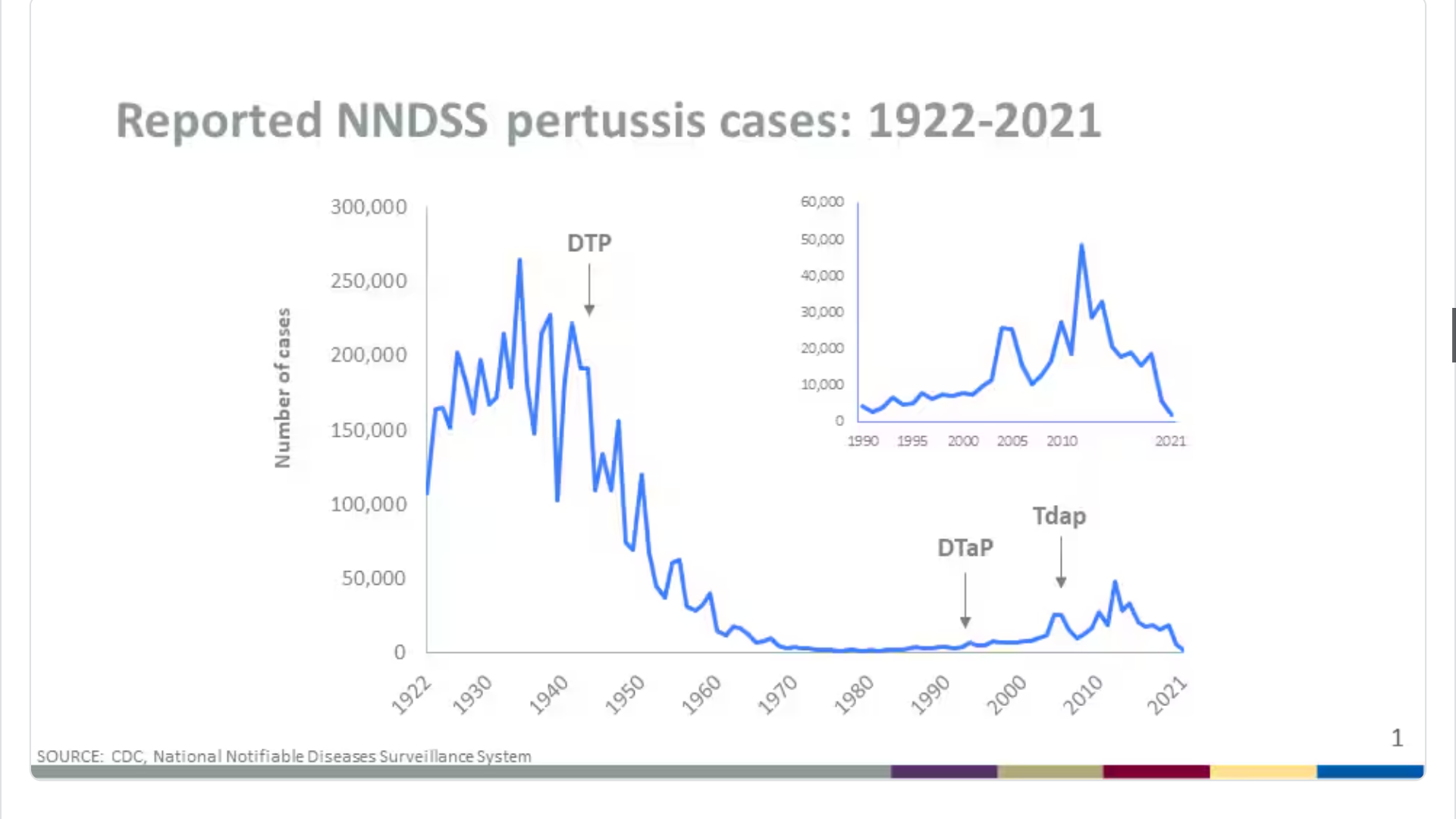
ILiAD Biotechnologies, LLC announced the selection of Emmes Group to conduct upcoming Phase III studies of its lead pertussis vaccine candidate, BPZE1.
As of June 24, 2024, ILiAD and Emmes Group are working to finalize the definitive agreement.
Multiple Phase III studies are expected to be conducted in North America, Central and South America, the U.K., and other global clinical sites.
BPZE1 is the leading next-generation pertussis vaccine designed to induce comprehensive and durable protection against B. pertussis infection (colonization) and disease (whooping cough). This vaccine is being developed to block B. pertussis from colonizing the nasal passages of adults and children, to protect adults and children from whooping cough, and to potentially prevent transmission, including transmission to infants.
"We are honored and pleased that ILiAD has selected Emmes Group as its partner to continue the clinical development of BPZE1. We look forward to working closely with ILiAD's clinical development team on this promising new vaccine, which could significantly reduce the transmissibility and incidence of B. pertussis, particularly in vulnerable populations," said Sastry Chilukuri, Chief Executive Officer of Emmes Group, in a press release.
While ILiAD is currently focused on developing a vaccine to protect adults and children and indirectly protect vulnerable infants, future development aims to immunize neonates directly. BPZE1 was developed at the Institut Pasteur de Lille (France) in the lab of Camille Locht, PhD and Nathalie Mielcarek, PhD.
According to the U.S. CDC, reported pertussis cases in 2024 increased across the U.S., indicating a return to more typical trends. Preliminary data show that more than three times as many cases have been reported to date in 2024 compared to the same time in 2023.