Search API
Most health experts say 'what happens in the Southern Hemisphere is a reliable indication of the next flu season in the United States.'
This opinion is based on the yearly circulating influenza viruses and flu shot composition.
Flu seasons in the Southern Hemisphere usually occur between April and September, compared with October through May in the north.
According to a new Morbidity and Mortality Weekly Report (MMWR) published today, the 2023-2024 influenza vaccines should protect people from severe hospitalizations.
On September 8, 2023, the U.S. Centers for Disease Control and Prevention (CDC) MMWR stated the adjusted vaccine effectiveness against severe acute respiratory infection hospitalization associated with any influenza virus during the 2023 Southern Hemisphere season was 51.9% (95% Confidence Interval 39.2%–62.0%).
Despite the encouraging data, fewer than 30% of persons identified in this CDC study were vaccinated against influenza in the five countries studied before their illness onset.
This news means the circulating influenza viruses were genetically similar to those targeted by the 2023–2024 Northern Hemisphere influenza vaccine available in the U.S.
Over 100 million flu shots have already been distributed this year.
In anticipation of the new flu season, the CDC recommends U.S. healthcare providers eagerly administer seasonal influenza vaccine to all eligible persons.
The findings in this CDC report are subject to at least five limitations, and no industry conflicts of interest were disclosed. Corresponding author: Ashley L. Fowlkes, [email protected].
Malaria was eliminated as a public health threat in the United States in the mid-1950s, recent locally-acquired cases refreshed the awareness of this mosquito-transmitted disease.
The species of Anopheles mosquitoes biologically capable of transmitting malaria have been found throughout the U.S.
The U.S. CDC published a Morbidity and Mortality Weekly Report on September 8, 2023, confirming eight malaria cases were reported in Florida (seven) and Texas (one) from May 18–July 17, 2023.
As of August 2023, no additional autochthonous P. vivax cases have been detected in Florida or Texas, and there has been no evidence of infected Anopheles mosquitoes since early June.
Although the risk for autochthonous malaria in the U.S. remains very low, U.S. clinicians need to consider a malaria diagnosis in patients with an unexplained fever, especially in areas where autochthonous malaria has been recently reported, wrote the CDC.
The recent cases underscore the potential for imported malaria cases from outbreak areas with competent vectors to produce local mosquito transmission of malaria parasites.
Before traveling internationally to areas where malaria is endemic, travelers should consult their healthcare provider regarding recommended malaria prevention measures, including potentially taking malaria prophylaxis.
Furthermore, malaria is preventable with vaccines available in Africa but not in the U.S.
Separately, the Pan American Health Organization (PAHO) called on Member States to maintain surveillance, early detection, and timely treatment of malaria cases in the Americas.
On September 7, 2023, the PAHO reported between 2022 and 2023, Argentina, Bahamas, and Jamaica reported sporadic cases of imported malaria and local transmission, including in areas where this disease had not previously been reported.
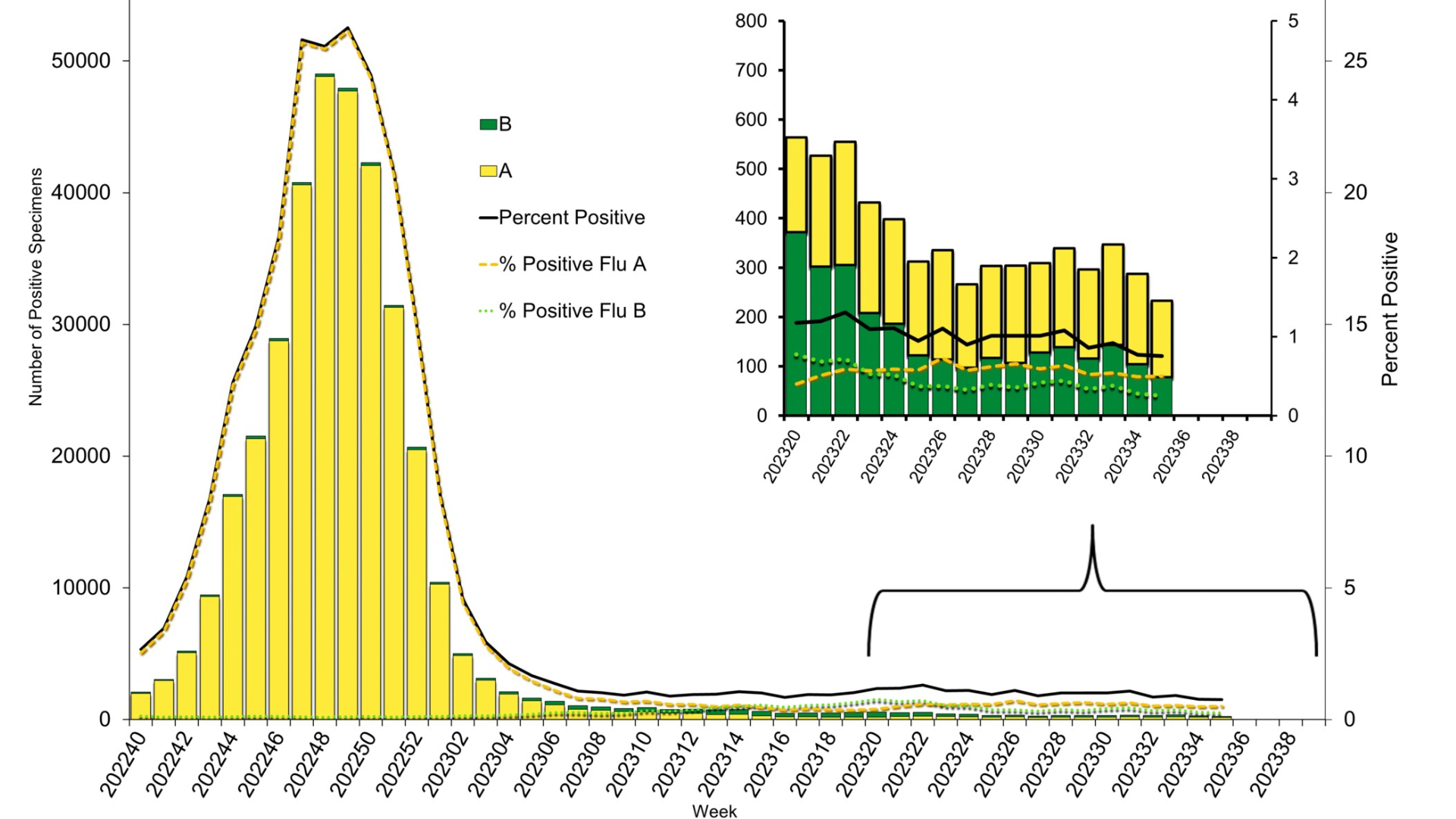
According to the U.S. Centers for Disease Control and Prevention (CDC) new report, very few influenza samples tested positive last week.
The CDC's FluView Week #35 report, published on September 8, 2023, data from clinical laboratories indicate 233 specimens tested positive for influenza, reflecting a 0.8% rate.
And public health laboratories reported only 41 positives out of 1,466 samples.
Nationwide, 1.8% of patient visits to healthcare providers last week were due to respiratory illness that included fever plus a cough or sore throat.
This data is increasing week over week, which can usually predict a flu outbreak is forecasted.
The unfortunate news is that influenza continues to cause related fatalities.
National Center for Health Statistics Mortality Surveillance data available on September 7, 2023, shows four death certificates listed influenza as an underlying or contributing cause of death.
Additionally, two influenza-associated pediatric deaths occurring during the 2022-2023 season were reported to the CDC during week #35.
A total of 174 influenza-associated pediatric deaths occurring during the 2022-2023 season have been reported to CDC. This is the most significant number of fatalities since the 2019-2020 flu season.
The CDC's new Director, Mandy K. Cohen, MD, MPH, recently recommended on X that people start getting their annual flu shot ahead of the 2023-2024 flu season.
'It's that time of year — I got my flu shot... Get yours to #FightFlu.'
A team of researchers at MIT today announced they are working on making mRNA vaccines produce a more robust immune response at a lower dose.
Published in the journal Nature Biomedical Engineering on September 7, 2023, this study showed how MIT researchers delivered COVID-19 antigen and the antigen to boost the immune response without needing a separate adjuvant.
The researchers’ tests also showed that the vaccine induced a strong immune response when delivered intranasally compared to the response elicited by traditional intramuscular vaccination.
In mice, intramuscular or intranasal administration of nanoparticles with the lead ionizable lipid and with mRNA encoding for the fusion protein (either the spike protein or the receptor-binding domain of SARS-CoV-2 increased the titres of antibodies against SARS-CoV-2 tenfold with respect to the vaccine encoding for the unadjuvanted antigen.
“With intranasal vaccination, you might be able to kill Covid (SARS-CoV-2) at the mucus membrane before it gets into your body,” commented Daniel Anderson, a professor in MIT’s Department of Chemical Engineering and the senior author of the study in an MIT News article.
“Intranasal vaccines may also be easier to administer to many people since they don’t require an injection.”
The researchers believe the effectiveness of other types of mRNA vaccines now in development, including vaccines for cancer, could be improved by incorporating similar immune-stimulating properties.
If further developed for use in humans, this type of mRNA vaccine could help to reduce costs, reduce the dosage needed, and potentially lead to longer-lasting immunity, wrote these researchers.
The National Institutes of Health and Translate Bio funded the research.

A joint team of Yale, University of Georgia, and Emory University scientists have received a $25 million government grant to develop an mRNA cancer vaccine.
On September 7, 2023, Carlos Salcerio with Yale News reported the research combines the team's expertise in mRNA and dendritic cells.
These researchers seek to develop synthetic mRNA that will carry instructions for cancer-specific antigens to dendritic cells.
By programming dendritic cells with synthetic mRNA, researchers can precisely instruct the immune system to target cancer-specific antigens without attacking healthy cells.
Healthy cells modified to include unrecognized proteins using mRNA might have otherwise been treated as a foreign entity.
The researchers recognized that vaccine breakthroughs often involve considerable complexities and difficulties.
"We're not naive.... we know how difficult this stuff is..... we have to pick our winners to move forward into the clinic.... but I would say that certainly there should be winners."

Three new outbreaks of cholera and/or Acute Watery Diarrhoea were recently reported from Uganda, Sudan, and the Republic of the Congo.
According to the World Health Organization (WHO) Edition #6, 28 countries have reported cholera cases since the beginning of 2023.
The WHO African Region remains the most affected region, with 16 countries reporting cholera outbreaks since the beginning of the year.
As of September 5, 2023, the overall capacity to respond to multiple and simultaneous outbreaks continues to be strained due to the global lack of resources, including shortages of oral cholera vaccines (OCV).
The WHO has pre-qualified three OCVs for use in 2023.
Based on the large number of outbreaks, their geographic expansion, and a lack of vaccines and other resources, WHO continues to assess the risk at the global level as very high.
In the U.S., there are very few cholera cases reported.
Since Ebola virus disease (EVD) impacts everyone in an outbreak area, another agency has approved a preventive vaccine for young children.
Merck today announced that the European Commission (EC) approved an expanded indication for the ERVEBO® vaccine for active immunization of individuals one year of age or older to protect against EVD caused by Zaire ebolavirus.
ERVENO was previously approved for use in the European Union for individuals 18 or older.
The U.S. FDA recently issued similar approval for young children.
ERVEBO is approved in the European Union, United Kingdom, United States, Canada, Switzerland, and ten countries in Africa.
ERVEBO is a live recombinant viral vaccine with a vesicular stomatitis virus backbone that protects people from Zaire ebolavirus. This vaccine does not protect people from the Sudan ebolavirus or the Marburg virus.
Dr. Eliav Barr, senior vice president, head of global clinical development, and chief medical officer, Merck Research Laboratories, commented in a press release on September 7, 2023, “When outbreaks of Ebola virus disease occur, they can quickly become a public health crisis. We are proud to play a role, alongside the global public health community, in helping to prepare for potential outbreaks of Zaire ebolavirus.”
In January 2021, Merck confirmed an agreement with UNICEF to establish the world’s first global Ebola vaccine stockpile to support future Zaire ebolavirus (EBOV) outbreak preparedness and response efforts.
Over 500,000 doses of ERVEBO have been delivered to the stockpile, administered by the International Coordinating Group on Vaccine Provision.
The initial EBOV case was confirmed in 1976 in the African countries of South Sudan and the Democratic Republic of Congo. Recent data suggest EBOV outbreaks may originate from human-to-human transmission instead of spillover events.
The U.S. Centers for Disease Control and Prevention published a list of EBOV Cases and Outbreaks as of August 2023.

While there are no approved human vaccines for Lyme disease, results from a new study show a booster dose can produce a strong immune response in children, adolescents, and adults.
France-based Valneva SE and Pfizer Inc. today announced positive pediatric and adolescent immunogenicity and safety data for their Lyme disease vaccine candidate, VLA15, when given as a booster.
The VLA15-221 Phase 2 clinical trial showed a strong anamnestic antibody response for all serotypes in pediatric (5 to 11 years of age) and adolescent participants (12 to 17 years of age), as well as in adults (18 to 65 years of age), one month after administration of a booster dose (month 19).
Depending on the primary vaccination schedule (month 0-2-6 or month 0-6), participants seroconverted after the booster dose, yielding seroconversion rates of 95.3% and 94.6% for all outer surface protein A (OspA) serotypes in all age groups, respectively.
Additionally, OspA antibody titers were significantly higher one month after the booster dose compared to one month after the primary schedule, with 3.3- to 3.7-fold increases (Geometric Mean Fold Rises) in adults, 2.0- to 2.7-fold increases in adolescents and 2.3- to 2.5-fold increases in children for all serotypes.
“Protection against Lyme disease is important for anyone who lives or spends time outdoors in areas where Lyme disease is endemic. This data from the VLA15-221 study is vital to improve our understanding of how vaccination may help to protect both adults and children from this potentially devastating disease,” commented Annaliesa Anderson, Ph.D., Senior Vice President and Head of Vaccine Research and Development at Pfizer, in a press release on September 7, 2023.
These results follow six-month antibody persistence data in children and adults reported for the VLA15-221 study in December 20222 and positive immunogenicity and safety data reported in April 20223.
Pfizer aims to submit a Biologics License Application to the U.S. Food and Drug Administration and a Marketing Authorisation Application to the European Medicines Agency in 2026, subject to positive Phase 3 data.

Moderna, Inc. today announced that clinical trial data from its research assay confirm its updated COVID-19 vaccine, which is pending approval by the U.S. Food and Drug Administration, generates an 8.7-fold increase in neutralizing antibodies in humans against BA.2.86 (Pirola), a new variant under monitoring.
The U.S. Centers for Disease Control indicates that the highly mutated BA.2.86 variant may be more capable of causing infection in people who previously had COVID-19 or were vaccinated with previous vaccines.
"These results demonstrate that our updated COVID-19 vaccine generates a strong human immune response against the highly mutated BA.2.86 variant. Taken together with our previously communicated results showing a similarly effective response against EG.5 and FL.1.5.1 variants, these data confirm that our updated COVID-19 vaccine will continue to be an important tool for protection as we head into the fall vaccination season," said Stephen Hoge, M.D., President of Moderna, in a press release on September 6, 2023.
Public health authorities are vigilantly monitoring the BA.2.86 variant, a highly mutated strain of the SARS-CoV-2 betacoronavirus with over 30 mutations as compared to prior Omicron strains.
Moderna's clinical trial data around its updated COVID-19 vaccine's effectiveness against BA.2.86 have been shared with regulators and submitted for peer review publication.

