Search API
The only imported quadrivalent seasonal influenza vaccine approved for use in individuals aged three years and older has been listed in 26 provinces and municipalities in China.
Clover Biopharmaceuticals, Ltd. today announced the launch of AdimFlu-S (QIS) in mainland China.
“The launch of AdimFlu-S in mainland China – our second commercialized product – strengthens Clover’s leading respiratory vaccine franchise and contributes to our financial sustainability and future growth,” said Joshua Liang, Chief Executive Officer and Executive Director of Clover, in a press release on September 12, 2023.
AdimFlu-S (QIS) is a quadrivalent split inactivated vaccine that can be used to prevent influenza. It contains hemagglutinin from four influenza virus strains (two A and two B).
This quadrivalent improves its ability to achieve high vaccine effectiveness, regardless of which influenza B strain becomes seasonally prevalent, compared to trivalent flu shot options, says the company.
AdimFlu-S gained approval from the China National Medical Products Administration in January 2022.
As of September 2023, over 100 flu shot candidates are in development globally.
Pfizer Inc. and BioNTech SE today announced that the U.S. Food and Drug Administration (FDA) approved the supplemental Biologics License Application (COMIRNATY 2023-2024 Formulation) for individuals 12 years and older.
And the FDA granted emergency use authorization for individuals six months through 11 years of age for the companies' Omicron XBB.1.5-adapted monovalent COVID-19 vaccine.
This decision follows guidance from the FDA's Vaccines and Related Biological Products Advisory Committee, which recommended an Omicron XBB.1.5-adapted monovalent COVID-19 vaccine for this fall and winter season.
"With today's decision, an updated vaccine will shortly become available that helps address multiple Omicron XBB-related sublineages, which currently account for the vast majority of COVID-19 cases globally," said Prof. Ugur Sahin, M.D., CEO and Co-founder of BioNTech, in a press release on September 11, 2023.
The approval of this season's COVID-19 vaccine is based on the full body of previous clinical, non-clinical, and real-world evidence supporting the safety and efficacy of the COVID-19 vaccines by Pfizer and BioNTech.
Further, the application included pre-clinical data showing this season's vaccine substantially improved responses against multiple Omicron XBB-related sublineages, including XBB.1.5, XBB.1.16, and XBB.2.3, compared to the Omicron BA.4/BA.5-adapted bivalent vaccine.
Additionally, pre-clinical (non-human) data demonstrate that serum antibodies induced by Omicron XBB.1.5-adapted monovalent COVID-19 vaccine, when compared to the Omicron BA.4/BA.5-adapted bivalent vaccine, effectively neutralize the recently emerged Omicron BA.2.86 (Pirola) and the globally dominant Omicron-related EG.5.1 (Eris) subvariant.
This season's COVID-19 vaccine will be available in pharmacies, hospitals, and clinics across the U.S. following a recommendation by the U.S. Centers for Disease Control and Prevention.
In Europe, the Comirnaty Omicron XBB.1.5 vaccine was recommended on August 30, 2023.
Also, on September 11, 2023, Moderna, Inc. announced the FDA approved the supplemental Biologics License Application for Spikevax®. Moderna's updated COVID-19 vaccine contains spike proteins for the XBB.1.5 sublineage of SARS-CoV-2 to help prevent COVID-19 in individuals 6 months of age and older.

To support an innovative bladder cancer vaccine candidate, ImmunityBio, Inc. today announced that it has executed financing transactions resulting in approximately $200 million of proceeds to the Company.
This financing includes an exchange into equity of current debt and a new convertible debt instrument from Nant Capital, LLC, an entity affiliated with Dr. Patrick Soon-Shiong, the Company's Founder, Executive Chairman, and Global Chief Scientific and Medical Officer.
With this new financing from Dr. Soon-Shiong, confirmed on September 11, 2023, includes the extension of the maturity date of the current debt.
ImmunityBio stated in a press release that it 'believes that it is well-positioned to fund its ongoing business operations and pre-commercialization efforts as it continues to drive toward a potential regulatory approval of N-803 plus BCG for BCG-unresponsive non-muscle invasive bladder cancer.'
"Our Company, scientists, physicians, and Board are grateful to Dr. Soon-Shiong for his continued financial support of our Company and its important mission, as well as for his involvement in our day-to-day operations," commented Richard Adcock, Chief Executive Officer and President of ImmunityBio.
"With this additional financing, we are well positioned to execute our commercialization plans in anticipation of the approval of N-803 plus BCG in bladder cancer."
"This funding will also help support the planned expansion of our current clinical trials and the opening of new studies to explore the untapped potential of N-803 and our other platforms across multiple indications."
N-803 Plus BCG is a therapy to treat adults with non-muscle-invasive Bladder Cancer carcinoma in situ with or without Ta/T1 papillary disease.
Its mechanism of action is direct specific stimulation of CD8+ T cells and NK cells through beta gamma T-cell receptor binding (not alpha) while avoiding T-reg stimulation.
The U.S. FDA is reviewing the Biologics License Application for N-803 plus BCG.

Since May 2022, a global outbreak of human mpox has proven to differ from the 2017–18 outbreak in Nigeria. The mpox strain responsible, Clade IIb, has mutated substantially, according to a study published in the peer-reviewed journal The Lancet Infectious Disease.
On September 4, 2023, this research study confirmed recent mpox cluster cases were described in individuals with presumed immunity through recent infection or vaccination.
These researchers found that of 37 men who have sex with men, seven individuals had mpox reinfections, and 29 individuals had mpox infections that occurred after two appropriately spaced JYNNEOS-Bavarian Nordic vaccine courses.
And one individual had an infection that met the criteria for both reinfection and infection after vaccination.
Those men, with an average of 36, with natural immunity after initial infection had a shorter disease course with less mucosal disease upon reinfection than with their initial infection.
Few lesions, minor mucosal disease, and minimal analgesia requirements characterized Mpox infections post-vaccination.
Overall, there were no deaths or bacterial superinfections, and all individuals were managed in the ambulatory clinic, with one hospital admission for a necrotizing neck lesion reported in the study.
As of May 2023, about 87,500 mpox cases and 141 related deaths were reported from 111 World Health Organization member countries.
Previous studies from the Netherlands, Spain, England, and the United States have described infections among children and adolescents during the recent mpox outbreak.
Globally, 1.3% of reported cases have been in children and adolescents.
This finding differed from 1970–2021 when mpox cases in Central Africa were predominately (54%–90%) reported in young children (ages 4–6).
For patients without known exposure to a person with mpox, various activities and interactions with others were reported in a separate study.
However, it was impossible to determine the likely source of infection for most of them, wrote these researchers. These highlight concerns about a potential mpox resurgence and have underscored the need to address critical knowledge gaps concerning immunity.
Previous mpox studies have been posted since 2022.

A Moderna Inc. funded study published on September 7, 2023, offered positive news ahead of the U.S. Centers for Disease Control and Prevention meeting of the Advisory Committee on Immunization Practices on September 12, 2023, at 10 a.m. ET.
This non-peer-reviewed preprint study of the ongoing phase 2/3 study of Moderna's mRNA-1273.815 monovalent (50-µg omicron XBB.1.5 spike mRNA) or mRNA-1273.231 bivalent (25-µg omicron XBB.1.5 and 25-µg omicron BA.4/BA.5 spike mRNAs) vaccines, administered as 5th doses to adults.
In this interim analysis, XBB.1.5-containing monovalent and bivalent vaccines elicited potent neutralizing responses against variants of the omicron XBB-lineage (XBB.1.5, XBB.1.6, XBB.2.3.2, EG.5.1, and FL.1.5.1) as well as the recently emerged BA.2.86 variant, in about 50 study participants.
And the safety profile of the XBB.1.5-containing vaccine was consistent with those of prior vaccines.
According to these researchers, these results overall indicate that the XBB.1.5-containing mRNA-1273.815 vaccine has the potential to provide protection against these emerging variants and support the Covid-19 vaccine update in 2023-2024 to a monovalent XBB.1.5-containing vaccine.
This study was not powered for a statistical comparison of the immune responses between the vaccine groups or vaccine efficacy. The corresponding author is Spyros Chalkias, MD Moderna, [email protected].
Previously, Moderna announced that clinical trial data from a research assay confirmed its updated COVID-19 vaccine generated an 8.7-fold increase in neutralizing antibodies in humans against BA.2.86 (Pirola).
Previously known as SpikeVax, this COVID-19 Vaccine is a Messenger RNA (mRNA) vaccine targeted against the SARS-CoV-2 betacoronavirus to prevent severe COVID-19. On December 18, 2020, the U.S. Food and Drug Administration issued an emergency use authorization.

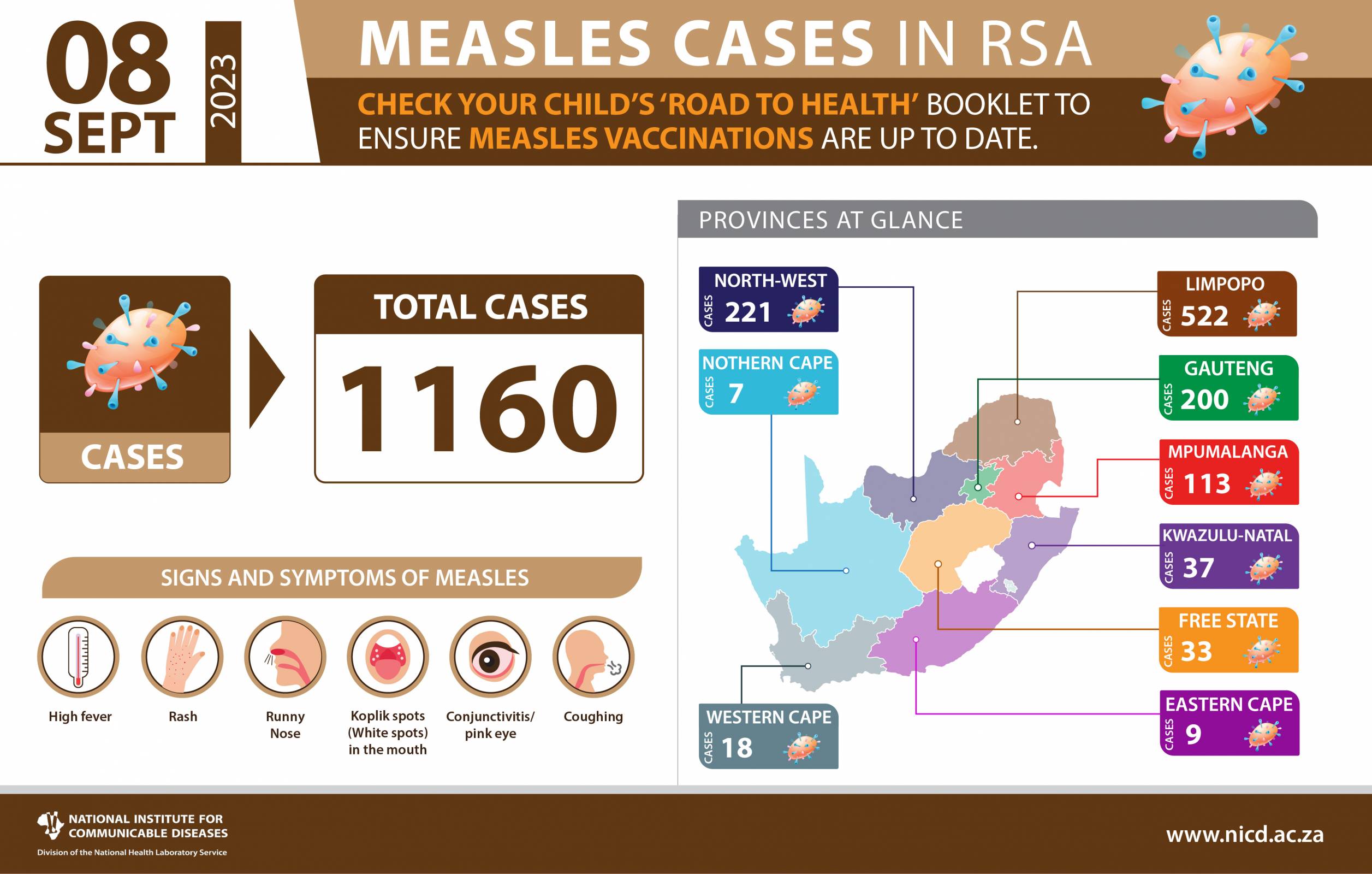
The Republic of South Africa's year-long battle against measles outbreaks continues in September 2023.
In the past weeks (week 34 up until week 35), 17 laboratory-confirmed measles cases were detected across the country from four of the eight provinces.
As of September 2, 2023, Gauteng province reported most of the cases (11), Limpopo reported three, Kwa-Zulu Natal reported two, and North West reported one case.
Measles is highly contagious. Around 90% of people who are not protected will become infected following exposure to the virus.
The National Institute for Communicable Diseases (NICD) criteria for declaring the measles outbreak was met in the Northern Cape province in week 15, the North West province in week 24, and the Free State province in week 25.
Since late 2022, the NICD has tested 6,816 serum samples for measles, of which 1160 (17%) were confirmed positive.
The NICD has implemented numerous vaccination programs throughout the measles outbreaks and continue in September 2023.
According to the U.S. Centers for Disease Control and Prevention (CDC), India leads all other countries over the past year with about 57,000 cases.
In addition, the CDC published a global Travel Health Notice on June 29, 2023, highlighting various measles outbreaks.
While most people have heard of norovirus outbreaks on cruise ships, this diarrhea-causing virus also impacts people on land.
The U.S. CDC's Morbidity and Mortality Weekly Report on September 8, 2023, highlighted about 27 cases of acute gastroenteritis among hikers on the Pacific Crest Trail in 2022, suggesting a possible norovirus outbreak.
The CDC wrote preventing future outbreaks will require fostering increased awareness of handwashing and the lack of effectiveness of alcohol-based hand sanitizers against norovirus and more frequent cleaning of public facilities.
These hikers and others are eagerly awaiting news of a norovirus vaccine.
On September 6, 2023, Vaxart, Inc. announced top-line data from the Phase 2b challenge study of its oral tablet monovalent norovirus vaccine candidate, VXA-G1.1-NN.
Vaccination with VXA-G1.1-NN led to a statistically significant reduction in infection rate, a non-statistically significant reduction in norovirus acute gastroenteritis, and a substantial reduction in viral shedding.
The oral vaccine candidate was also safe and well tolerated with no vaccine-related serious adverse events.
Dr. James F. Cummings, Vaxart's Chief Medical Officer, commented in a press release, "The magnitude of the reduction in shedding could have an impact on transmission and may have important public health benefits, as norovirus spreads rapidly among people gathering in large numbers, including settings such as daycare centers, nursing homes, and workplaces, and may reduce the potential spread of the infection to families at home."
"The results of this study highlight the potentially distinctive profile of mucosal vaccination and the potential that our oral pill vaccines may have in protecting against infection and blocking transmission – potential benefits that have also been seen with our influenza vaccine," added Dr. Cummings.
Norovirus is the leading cause of acute viral gastroenteritis in all age groups in the U.S. On average, norovirus causes 19 to 21 million cases, infects 15% of all children under age 5, and leads to 465,000 emergency department visits, 109,000 hospitalizations, and 900 deaths, says the U.S. CDC.
As of September 9, 2023, norovirus vaccine candidate news is posted by Precision Vaccinations.