Search API
The Ministry of Health, Welfare and Sport of the Netherlands recently notified the World Health Organization (WHO) of a laboratory-confirmed human case of infection with a swine-origin influenza A(H1N1) variant virus.
This is the first human swine flu case reported in the Netherlands this year.
As of September 7, 2023, there were no symptomatic contacts of this case; the person has recovered, and no further detections have been reported.
Therefore, the likelihood of community-level spread among humans and/or international disease spread through humans is considered 'low,' says the WHO.
Zoonotic influenza infections are caused by germs, bacteria, parasites, and fungi, says the U.S. Centers for Disease Control and Prevention (CDC). These germs cause illnesses in people, birds, and animals, ranging from mild to severe infections.
When a swine influenza virus is detected in a person, it is called a "variant influenza virus."
As of September 14, 2023, the CDC says annual 'flu shots' do not prevent zoonotic influenza infections such as swine flu (H3N2) or avian influenza (Bird Flu).

The European Medicines Agency (EMA) today announced its Human Medicines Committee (CHMP) has recommended authorizing an adapted Spikevax vaccine targeting the Omicron XBB.1.5 subvariant.
In its decision to recommend the authorization on September 14, 2023, the CHMP considered all the available data on Spikevax and its other adapted vaccines.
Known as Spikevax XBB.1.5, this vaccine prevents COVID-19 in adults and children from six months of age.
In line with previous recommendations by EMA and the European Centre for Disease Prevention and Control, adults and children from 5 years of age who require vaccination should have a single dose, irrespective of their COVID-19 vaccination history.
Children from 6 months to 4 years of age may have one or two doses depending on whether they have completed a primary vaccination course or have had COVID-19.
The CHMP also considered data from a study in which adults were given Spikevax XBB.1.5 as a booster.
The study showed that the vaccine produced an immune response against the Omicron XBB.1.5 subvariant, as measured by a rise in antibodies against this strain.
The vaccine also produced an immune response against several other strains of the betacornavirus that causes COVID-19, including the currently circulating Omicron XBB.1.16 subvariant.
The EMA has sent the CHMP’s recommendation to the European Commission for an EU-wide legally binding decision.
SpikeVax has recently been approved in Canada and the United States.

Moderna Inc.'s respiratory vaccine franchise is targeting an approximately $30 billion annual market, comprised of a $15 billion COVID-19 market, a $10 billion RSV market, and a $6 billion flu market, with the potential for growth with more effective vaccines.
According to Modern'a press release on September 13, 2023, the Company's respiratory products sales in 2027 are expected to be in the range of $8 billion to $15 billion, depending on certain variables.
"With today's positive Phase 3 flu results, along with previous results in COVID and RSV, we are now three for three on advancing respiratory disease programs to positive Phase 3 data," commented Stéphane Bancel, Chief Executive Officer of Moderna, in a press release.
Modera's press release specifically highlighted its Seasonal Influenza Vaccine business today.
The mRNA-1010 vaccine candidate has demonstrated an acceptable safety and tolerability profile across all clinical trials to date, including three Phase 3 trials (P301, P302, P303), and independent data and safety monitoring boards have raised no safety concerns.
In an interim analysis of the P303 study,mRNA-1010 met all co-primary endpoints across all four A and B strains (A/H1N1, A/H3N2, influenza B/Yamagata, B/Victoria).
Higher HAI geometric mean titers and seroconversion rates were observed for all four strains compared to a licensed comparator (Fluarix).
And local and systemic solicited adverse reactions were similar to those reported in previous mRNA-1010 studies.
Furthermore, improved immunogenicity was observed across age groups and, importantly, was seen in older adults.
mRNA-1010 also elicited higher HAI titers against A/H1N1, A/H3N2, B/Victoria, and comparable titers to B/Yamagata compared to Fluzone HD in a separate Phase 1/2 head-to-head study.
As the previous P302 efficacy study has not accrued its target case numbers by the end of the most recent season, the Company would need to enroll in a second season to accrue enough cases.
In light of P303 meeting all its primary endpoints, the Company has decided not to enroll in a second season in the P302 study.
The Company continues to advance a portfolio of influenza vaccine candidates that include additional HA antigens for broader coverage of circulating influenza A strains (mRNA-1011 and mRNA-1012) and candidates that incorporate both HA and neuraminidase antigens to target multiple proteins involved in the influenza virus lifecycle to reduce the potential of viral antigenic escape (mRNA-1020 and mRNA-1030).
As of early September 2023, over 100 million influenza vaccines have been distributed in the U.S., preparing for the 2023-2024 flu season.

When the U.S. Food and Drug Administration (CDC) approved and authorized newer mRNA COVID-19 vaccines yesterday, they also withdrew the current mRNA vaccine use in the U.S.
The FDA stated in a September 11, 2023, press release that the bivalent Moderna and Pfizer-BioNTech COVID-19 vaccines are no longer authorized.
The FDA approved and authorized the emergency use of updated mRNA COVID-19 vaccines formulated to more closely target currently circulating variants and provide better protection against serious consequences of COVID-19, including hospitalization and death.
These enhanced mRNA vaccines are unavailable at health clinics or pharmacies as of September 12, 2023.
This news indicates Novavax Inc.'s protein-based COVID-19 vaccine is the only one in use until the new vaccines arrive.
Furthermore, the U.S. CDC's Advisory Committee on Immunization Practices did vote affirmatively today on its recommendation for the new mRNA vaccines. The final authorization step is for the CDC's Director to issue her approval.

Replicate Bioscience, a clinical-stage company pioneering its novel self-replicating RNA (srRNA) technology to overcome the limitations of current mRNA modalities, today announced the dosing of the first participant in a Phase 1 trial of its RBI-4000 vaccine for the prevention of rabies.
The Phase 1 trial will evaluate the safety, tolerability, and immunogenicity of RBI-4000 in 84 participants in the U.S.
In preclinical studies, intramuscular administration of RBI-4000 provided durable protection against the rabies virus, inducing antibody production and robust virus-specific T cells.
"Replicate's srRNA technology offers the potential for more robust and durable immune responses and improved tolerability at lower doses than existing mRNA approaches," commented Zelanna Goldberg, M.D., CMO of Replicate, in a press release on September 12, 2023.
"In infectious disease specifically, our srRNA technology unlocks opportunities to effectively address more complex infectious disease indications and allows us to rapidly develop vaccine candidates to treat or prevent illness — a crucial capability for future pandemic readiness."
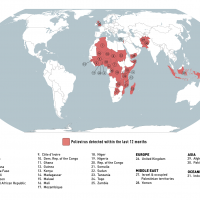
According to the World Health Organization, Rabies is a vaccine-preventable viral disease found in more than 150 countries and territories. Rabies vaccination can prevent infections before and after exposure to the rabies virus.
Various rabies vaccines are approved and available in 2023.
While the U.S. FDA issued various authorizations for mRNA COVID-19 vaccines yesterday, the only protein-based COVID-19 vaccine was included in the Advisory Committee on Immunization Practices (ACIP) meeting on September 12, 2023.
Dr. Filip Dubovsky, President Research & Development, Novavax Inc., presented updated data supporting the Novavax XBB.1.5 Vaccine.
This ACIP presentation included, but is not limited to, the following:
- Non-human primate data demonstrates that Novavax vaccine technology induces broadly neutralizing antibodies with primary vaccination,
- Neutralizing responses against XBB subvariants achieve levels comparable to homologous XBB.1.5 response,
- Consistent responses against all variants including emerging XBB subvariants.
In summary, Dr. Dubovsky stated during today's webinar that non-clinical data supports the use of the Novavax XBB.1.5 vaccine, there are robust neutralizing responses against XBB subvariants and data that generate a polyfunctional Th1-biased CD4+ cellular immune response against XBB subvariants.
Novavax COVID-19 vaccine brands include Nuvaxovid, CovoVax, and NVX-CoV2373, and has been distributed in about 40 markets as of September 2023.