Search API
According to an email from an Administration for Strategic Preparedness and Response spokesperson on July 15, 2024, the first H5N1 avian influenza vaccine doses are scheduled to roll off the line next week, with the remaining doses following through August 2024.'
Other steps besides filling and finishing the vaccine, such as policy and regulatory decisions, must occur before the vaccine is released for public use.
Avian influenza vaccination has not been recommended for any population segment, and the U.S. government continues to monitor the situation.
Currently, the CDC evaluates the overall risk to human health as low.
On June 27, 2024, the U.S. Centers for Disease Control and Prevention confirmed during its vaccine committee meeting that an avian vaccination program was inactive.
As of July 17, 2024, the U.S. and European (Finland) governments have approved various avian influenza vaccines and recently awarded millions of dollars in vaccine candidate development contracts. Previous U.S. FDA-approved avian vaccines have reported measurable side effects.

Diakonos Oncology Corporation announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for the company’s unique dendritic cell vaccine (DCV) for pancreatic ductal adenocarcinoma.
On July 15, 2024, the Houston-based company confirmed that DCVs are made with a patient’s dendritic cells and a tumor sample.
These highly differentiated double-loaded dendritic cell vaccines activate robust cytotoxic TH1 cell signaling pathways that initiate a natural immune response to target and eliminate cancer cells. This is achieved without any genetic modification of the patient’s immune cells, which greatly simplifies the manufacturing process and significantly reduces costs compared to leading cell therapy approaches.
“This second FDA Fast Track designation of our autologous dendritic cell vaccines for pancreatic cancer is another acknowledgment of the incredible potential of this innovative immunotherapy for treating the most deadly cancers,” said Mike Wicks, Diakonos CEO, in a press release.
Pancreatic ductal adenocarcinoma is the most common pancreatic cancer. In 2024, an estimated 51,750 people will die, and 66,440 will be newly diagnosed.
FDA Fast Track designation is intended to speed the development and review of drugs that show early clinical promise in treating severe or life-threatening conditions.

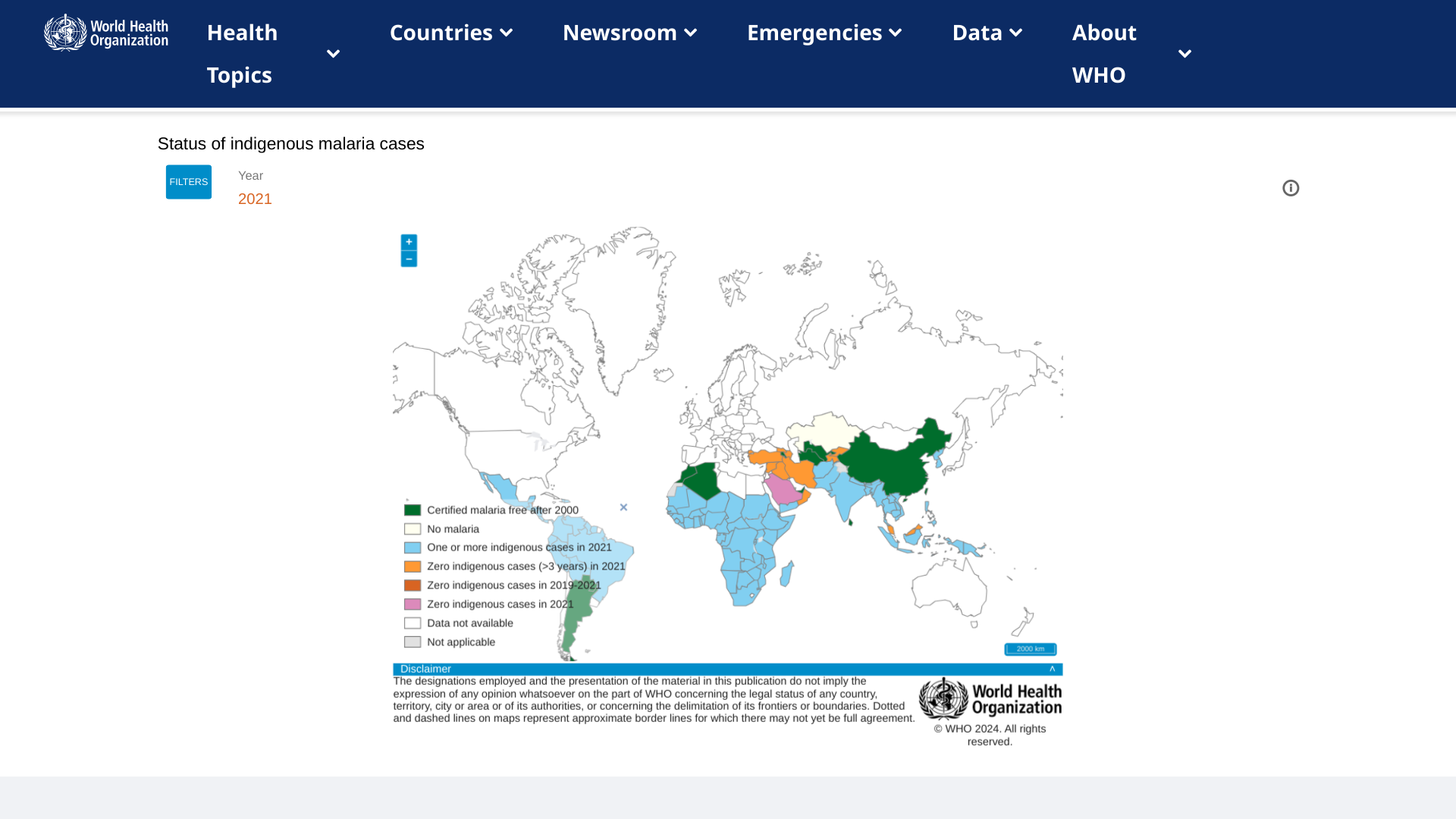
Today marks the official rollout of the newly approved R21/Matrix-M™ malaria vaccine, co-developed by the University of Oxford and Serum Institute of India (SII), which has committed to producing 100 million vaccines.
This malaria vaccine utilizes Novavax’s Matrix-M™ adjuvant technology.
The first official vaccination is scheduled for July 15, 2024, in Abidjan, Côte d’Ivoire, and it will be subsequently introduced in 38 districts across the country.
According to a press release, 15 African countries will introduce malaria vaccines with Gavi support in 2024. These countries plan to reach around 6.6 million children with the malaria vaccine in 2024 and 2025.
John Jacobs, President and Chief Executive Officer of Novavax Inc., commented, "The introduction of the R21/Matrix-M™ malaria vaccine in Côte d'Ivoire marks a breakthrough in the fight to protect vulnerable children against a leading cause of death across the region while reinforcing our mission to create innovative vaccines that improve public health."
"Novavax is proud of the contribution of our Matrix-M™ adjuvant in this vaccine and in making this moment possible, and value our continued collaboration with the University of Oxford and SII, as well as the lifesaving work of WHO, Gavi, and UNICEF.”
R21/Matrix-M is a low-dose, highly effective, and affordable vaccine that can be manufactured quickly and scale. Ghana, Nigeria, Burkina Faso, and the Central African Republic have approved the new vaccine, and many others are preparing to receive shipments.
Malaria vaccines are currently unavailable in the United States.
Novavax, based in Gaithersburg, MD., U.S., promotes improved health by discovering, developing, and commercializing innovative vaccines to help protect against serious infectious diseases.
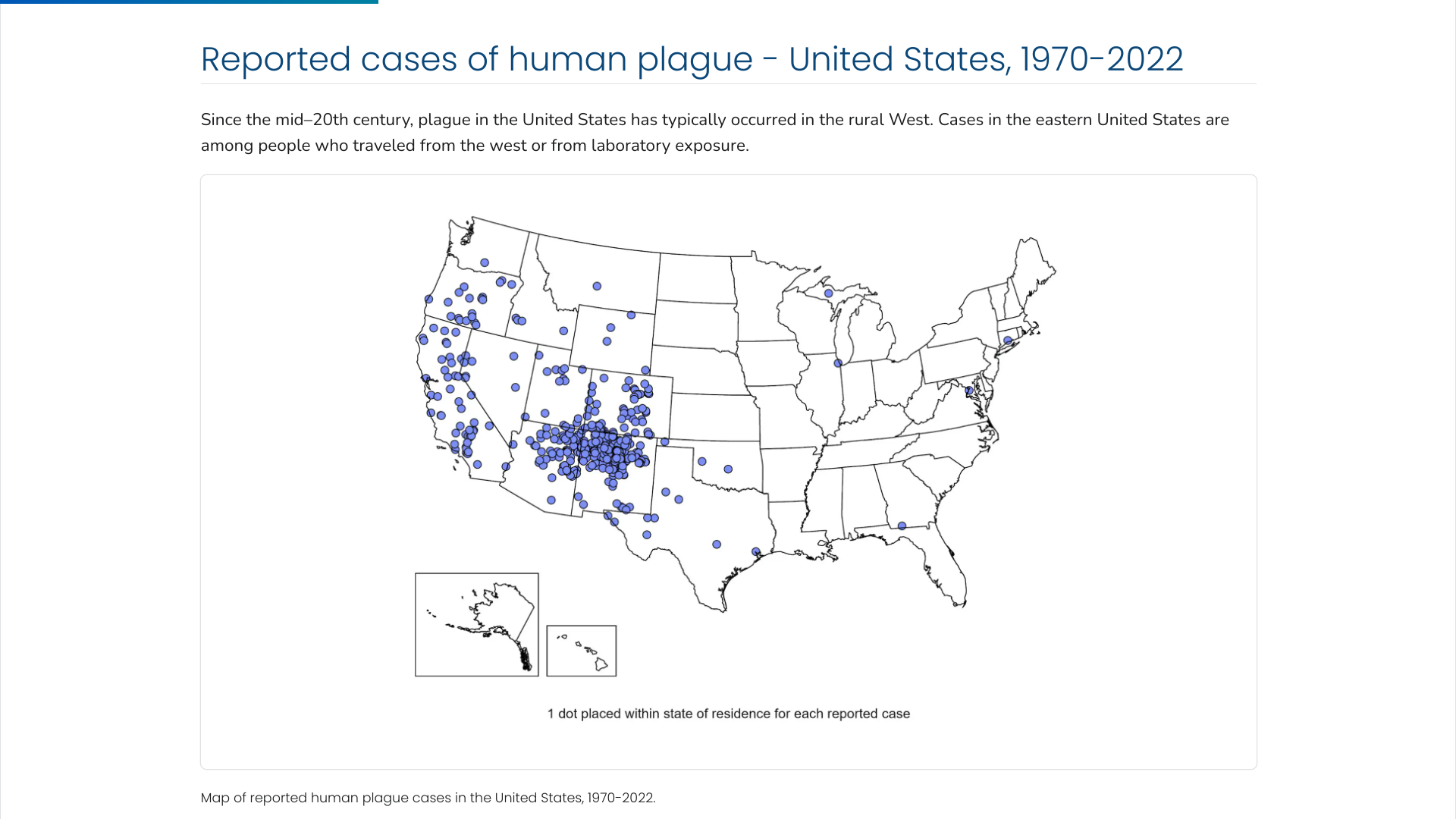
The U.S. Centers for Disease Control and Prevention (CDC) says the Plague was first introduced into the United States in 1900. The plague bacterium (Yersinia pestis) is transmitted by fleas and cycles naturally among wild rodents.
Over the decades, the Plague spread from urban rats to rural rodent species and became entrenched in many areas of the western U.S.
Almost all of the cases reported in the last 20 years have occurred among people living in small towns and villages or agricultural areas rather than in larger towns and cities, says the CDC.
As of 2024, the CDC estimates that seven human cases of Plague occur in the U.S. each year.
Recent plague cases include the Pueblo Department of Public Health and Environment confirming a human case of Plague in a Pueblo County resident on July 9, 2024.
And in February 2024, health officials in Oregon reported a case of bubonic Plague in a resident who they said likely contracted it from a pet cat.
Globally, the most human plague cases since the 1990s have occurred in Africa.
From a prevention perspective, plague vaccines are no longer available in the U.S. However, plague vaccine candidates are in development but are not expected to be commercially available in the immediate future.
In March 2023, the first mRNA-based, lipid nanoparticle vaccine was found effective against lethal bacteria in mice.
Tiba Biotech LLC today announced its new partnership with the Biomedical Advanced Research and Development Authority (BARDA) through the initiation of an EZ-BAA contract to develop groundbreaking therapeutics against influenza.
The U.S. $749,999 BARDA contract supports early-stage therapeutic platform development for the Flexible and Strategic Therapeutics program.
This BARDA initiative aims to advance cutting-edge therapeutic platform technologies, such as RNA-mediated interference, which could be rapidly deployed in response to emerging viral threats. The therapeutic will target the highly conserved viral nucleoprotein and will be delivered via Tiba’s RNABLTM platform.
Tiba Biotech’s initial focus will be developing a prototype RNAi-based therapeutic for H1N1 influenza (swine flu), a type of influenza A virus.
Every year, there are rare, sporadic human infections with influenza viruses that usually circulate in pigs and not people, says the U.S. CDC.
On June 28, 2024, the CDC reported the second and third U.S. human infections in 2024.
This initiative is a natural extension of Tiba’s ongoing work combating influenza pandemic threats, most notably in the form of a novel multi-antigen mRNA-based H7N9 flu vaccine funded under a Phase II SBIR award from the National Institutes of Health and ongoing collaborations with the Collaborative Influenza Vaccine Innovation Centers.
Tiba was also recently accepted into BLUE KNIGHT™, a joint initiative between Johnson & Johnson Innovation—JLABS and BARDA. The initiative aims to harness innovation to combat future known and unknown health threats.

A virus-like particle (VLP) based vaccine candidate in development to prevent moderate-to-severe acute gastroenteritis (AGE) in infants caused by norovirus infection is being discontinued.
This is unfortunate news since the health burden of norovirus falls disproportionately on young children and older adults.
HilleVax, Inc. today announced topline data results from NEST-IN1, a Phase 2b, randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy, safety, and immunogenicity of the HIL-214 vaccine candidate in infants of approximately five months of age at the time of initial vaccination at sites in the United States and Latin America.
In the NEST-IN1 study, there were 51 primary endpoint events, with 25 in the vaccine arm (n=1,425) and 26 in the placebo arm (n=1,399), resulting in a vaccine efficacy of 5% (95% confidence interval; -64%, 45%).
The study did not meet its primary efficacy endpoint against moderate or severe AGE events due to GI.1 or GII.4 norovirus genotypes. And no clinical benefit was observed across secondary endpoints. The company plans to discontinue further development of HIL-214 in infants.
HIL-214 did exhibit a safety and immunogenicity profile consistent with what was observed in the prespecified analysis of the first 200 subjects in NEST-IN1 and previously reported studies.
“We are disappointed that the NEST-IN1 study did not meet its primary efficacy endpoint,” said Rob Hershberg, MD, PhD, Chairman and Chief Executive Officer of HilleVax, in a press release on July 8, 2024.
“While HIL-214 previously showed clinical benefit in adults, NEST-IN1 was the first efficacy study conducted in infants for a norovirus vaccine candidate. We believe the efficacy in the infant setting may have been impacted by the appearance of multiple emerging GII.4 strains in this trial.”
The company stated it is exploring the potential for continued development of HIL-214 and HIL-216 in adults, HilleVax’s Phase 1 ready vaccine candidate.
Currently, there are no U.S. FDA-approved norovirus vaccines.
Globally, norovirus is estimated to result in approximately 700 million cases of AGE and 200,000 deaths per year, resulting in over $4 billion in direct health system costs and $60 billion in societal costs per year.
The U.S. CDC publishes information about gastrointestinal illness outbreaks on cruise ships in the Vessel Sanitation Program during 2024, including details and actions taken in response.

GSK plc recently announced a restructuring of its collaboration agreement with CureVac N.V. Under the new agreement, GSK will focus on the development of mRNA vaccines for influenza and COVID-19 while withdrawing from other infectious disease projects.
As part of the revised contract reported on July 3, 2024, GSK will pay CureVac €400 million (approx. $430 million) upfront. Additionally, GSK has committed to providing up to $1.13 billion in development, regulatory, and sales milestone payments and offering tiered royalties.
Tony Wood, GSK's chief scientific officer, said in a press release, “We are excited about our flu/COVID-19 programs and the opportunity to develop best-in-class mRNA vaccines to change the standard of care. With this new agreement, we will apply GSK’s capabilities, partnerships, and intellectual property to CureVac’s technology to deliver these promising vaccines at a pace.”
This new deal will replace all previous financial terms from the original agreement. In exchange for these payments, GSK will secure full global rights to develop and commercialize CureVac’s investigational mRNA vaccines for influenza and COVID-19, including combination formulations.
Currently, the partners have seasonal flu and COVID-19 shots in Phase II development and an avian flu candidate in Phase I. Both companies believe that these candidates have best-in-class potential.
Completion of the new agreement remains subject to certain antitrust and regulatory approvals and customary closing conditions. The original collaboration between CureVac and GSK was initiated in July 2020.
CureVac is a multinational biotech company founded in 2000 to advance the field of messenger RNA (mRNA) technology for application in human medicine.



