Search API
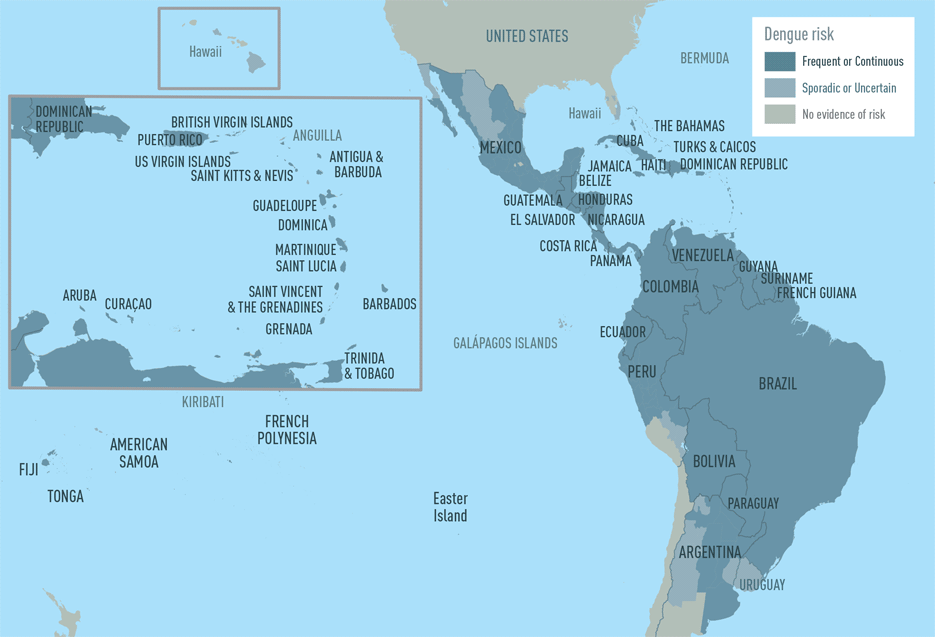
Some countries are reporting increased dengue outbreaks in Central and South America, Mexico, and the Caribbean during 2023. Dengue can become severe within a few hours. Severe dengue is a medical emergency, usually requiring hospitalization.
The U.S. Center for Disease Control and Prevention (CDC) issued a Level 1 Travel Health Notice on September 25, 2023, to alert international travelers of this mosquito-transmitted health risk.
According to the CDC, dengue is endemic in about 125 countries.
The countries listed below have recently reported higher-than-usual dengue cases, and travelers visiting these countries may be at increased risk of catching this serious disease.
Argentina
Colombia
Cuba
Guadeloupe
Guatemala
Jamaica
Martinique
Nicaragua
Panama
Peru
In the U.S., 44 jurisdictions have reported about 880 dengue cases in 2023. The majority of these dengue cases were detected in Florida and Puerto Rico.
Since October 2022, two dengue vaccines have been authorized in various countries.
A novel rabies vaccine candidate designed to produce a more robust immune response in an accelerated timespan compared to existing rabies vaccines today announced it has progressed to late-stage clinical research.
YS Biopharma Co., Ltd. confirmed on September 26, 2023, that it has enrolled the first subject in its Phase 3 clinical trial of the PIKA Rabies Vaccine candidate.
The trial will assess the safety, immunogenicity, and lot-to-lot consistency of the PIKA Rabies Vaccine and is expected to include an estimated 4,500 subjects.
Dr. Muhammad Ahmad, the Principal Investigator at Central Park Teaching Hospital in Lahore, Pakistan, where the first subject has been enrolled, commented in a press release, "This marks an important step forward in our collective efforts to develop a novel and powerful vaccine which leverages recent immunological advancements."
"We are optimistic that these results will help shape the future of vaccine interventions and aid in treating a pressing global public health issue."
This experimental vaccine may add clinical value to the currently approved rabies vaccines as sporadic breakthrough infections (i.e., rabies cases in people vaccinated) have been reported, according to The Lancet Infectious Disease in December 2022.
Rabies is a viral disease characterized by an almost 100% mortality rate upon the onset of clinical symptoms.
The virus is responsible for approximately 59,000 human fatalities annually in over 150 countries, primarily in Asia and Africa.
In the U.S., bites from bats, not dogs, are the leading source of rabies infections.
The U.S. CDC says before visiting high-risk rabies destinations, discuss vaccination options with a healthcare provider.

The World Health Organization (WHO) Director-General, Dr. Tedros Adhanom Ghebreyesus, recently launched the TB Vaccine Accelerator Council to facilitate the development, licensing, and using new Tuberculosis (TB) vaccines.
The 100-year-old Bacille Calmette-Guérin (BCG) is the only licensed TB vaccine, with over ten versions available globally.
While it provides moderate efficacy in preventing severe forms of TB in infants and young children, it does not adequately protect adolescents and adults, who account for the majority (>90%) of TB transmission globally, said the WHO on September 22, 2023.
In a press release, Dr. Tedros Adhanom Ghebreyesus commented, "Today, we have knowledge and tools they could only have dreamed of."
"The political declaration countries approved today, and the targets they have set, are a commitment to use those tools, and develop new ones, to write the final chapter in the story of TB."
The Council aims to identify innovative sustainable financing, market solutions, and partnerships across public, private, and philanthropic sectors. It will leverage various agencies to strengthen commitment and actions for novel TB vaccine development and access.
The U.S. Centers for Disease Control and Prevention (CDC) reported that TB outbreaks increased by 5% in 2022, with 60 U.S. states, the District of Columbia, and territories provisionally reporting 8,300 TB cases last year.
According to the CDC, TB is caused by Mycobacterium tuberculosis. The bacteria usually attack the lungs, but TB bacteria can attack any body part.
As a result, two TB-related conditions exist: latent TB infection and TB disease. If not treated properly, TB disease can be fatal.
In the U.S., Merck's TICE BCG vaccine has limited availability.

The U.S. Centers for Disease Control and Prevention (CDC) recently announced it had granted 13 funding awards to establish a first-of-its-kind national network, the Outbreak Analytics and Disease Modeling Network (OADMN).
The OADMN's goal is to improve speed, accuracy, and use of data & analytics during health emergencies, which is an important step towards ensuring Americans have the information they need to keep themselves and their families safe during outbreaks.
Many of these awardees are leading a consortium of collaborators to design, prototype, test, and scale up advances in data modeling tools and technology that can be used to support public health decision-makers at all levels of government.
"The collaboration with our public health, private, and academic partners over the last year to advance the science of disease forecasting and deliver decision support to leaders has been instrumental in improving outbreak response," said Dr. Dylan George, Director, Center for Forecasting and Outbreak Analytics, in a press release on September 22, 2023.
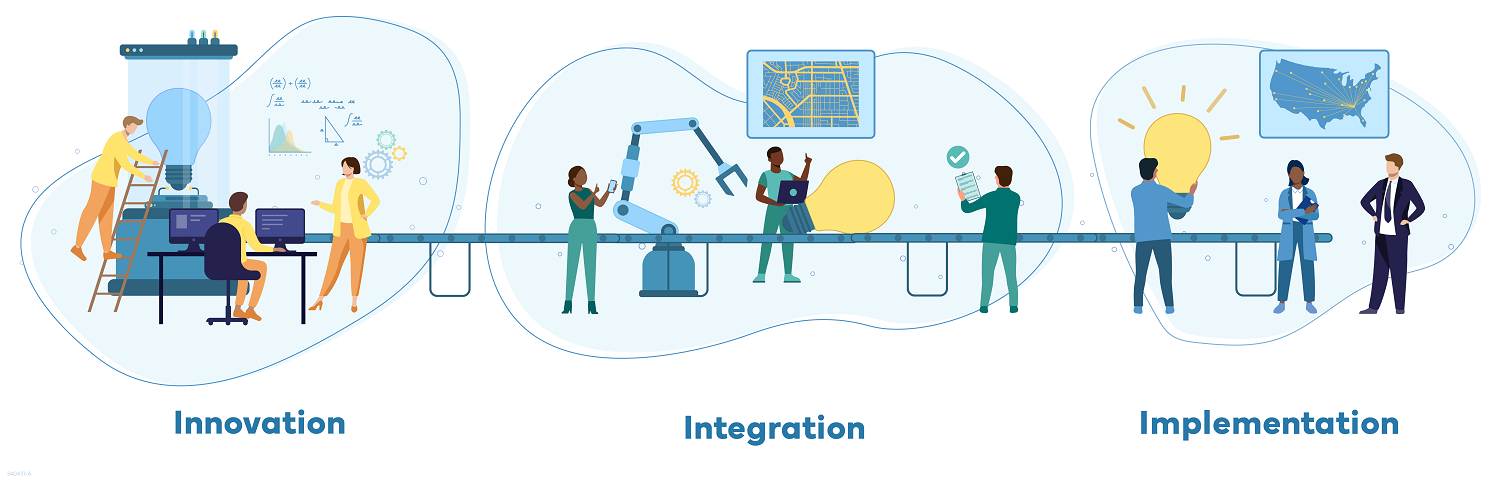
The grantees will be organized into three categories of performance, supporting three priority program actions:
- Innovators: these grantees will develop methods, tools, technologies, and other products to support emergency response.
- Integrators: these grantees will take lessons learned and techniques from Innovators and test them in small-scale deployments.
- Implementors: these grantees will take tested best practices and scale up to larger jurisdictions and partners.
The funding 13 recipients comprising OADMN in September 2023 include:
Emory University
Northeastern University
The University of North Carolina at Chapel Hill
Kaiser Permanente Southern California
Carnegie Mellon University
University of Michigan School of Public Health
University of California, San Diego
University of Minnesota
Clemson University
University of Utah
International Responder Systems
The University of Texas at Austin
The Johns Hopkins University
Note: The OADMN effort is in addition to the U.S. government's Disease X initiative.
During last week's Advisory Committee on Immunization Practices (ACIP) meeting, an updated adult, adolescent, and infant vaccination schedule was presented.
On September 22, 2023, the CDC's Sarah Schillie, MD, MPH, MBA CAPT, US Public Health Service, presented a draft Addendum to the 2023 Immunization Schedules.
This Addendum contains ACIP recommendations that occurred after the 2023 schedule was published earlier this year.
For 2023, two separate vaccination schedules with multiple sections summarize and aid in implementing the approved ACIP policy.
This is the most up-to-date vaccine recommendation for COVID-19, RSV, Influenza, pneumococcal, polio, and Mpox vaccines.
The CDC plans to release 2023 Immunization Schedules with Addenda this week. With this addition, all ACIP recommendations will formally be part of the Immunization Schedules.

BioArctic AB and Eisai announced today that LEQEMBI® Intravenous Infusion had been approved in Japan as a treatment for slowing the progression of mild cognitive impairment and mild dementia due to Alzheimer's disease (AD).
LEQEMBI is a humanized immunoglobulin gamma 1 monoclonal antibody (mAbs) directed against aggregated soluble and insoluble forms of Aβ.
LEQEMBI is the first and only approved treatment shown to reduce the rate of disease progression and slow cognitive and functional decline by selectively binding to and eliminating the most toxic Aβ aggregates that contribute to neurotoxicity in AD.
Japan is the second country to approve LEQEMBI, following the Food and Drug Administration (FDA) approval in the U.S. in July 2023.
"The approval of LEQEMBI in Japan is another important step in the fight against Alzheimer's disease," said Gunilla Osswald, CEO of BioArctic, in a press release on September 25, 2023.
Eisai serves as the lead of LEQEMBI development and regulatory submissions globally, with both Eisai and Biogen co-commercializing and co-promoting the product and Eisai having final decision-making authority.
BioArctic has the right to commercialize lecanemab in the Nordic region, and currently, Eisai and BioArctic are preparing for joint commercialization in the region.
According to the Alzheimer's Association, AD is a degenerative brain disease caused by complex brain changes following cell damage, leading to dementia.
As of September 2023, The U.S. FDA, the European Medicines Agency, and the United Kingdom's NHS have not authorized preventive AD vaccines.

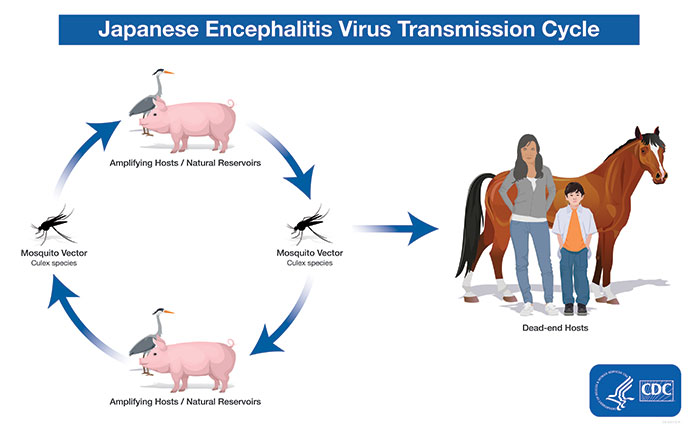
A specialty vaccine company today announced the signing of a $32 million contract with the United States (U.S.) Department of Defense (DoD) for the immediate supply of its Japanese encephalitis (JE) vaccine, IXIARO®.
Under this new one-year contract with Valneva SE, the DoD can purchase additional doses for the next twelve months.
IXIARO® is the only JE vaccine approved for adults by the U.S. Food and Drug Administration (FDA).
Dipal Patel, Chief Commercial Officer of Valneva, commented in a press release on September 25, 2023, "We are excited to continue our long-term relationship with the DoD."
"The U.S. military has trusted IXIARO® for over ten years to help protect military personnel, their families, civilian government service personnel, and government contractors from this potentially deadly disease."
Japanese encephalitis is a deadly infectious disease found mainly in Asia. The disease is endemic in Southeast Asia, India, and China, a region with more than three billion population.
About 70,000 cases of JE are estimated to occur in Asia each year.
JE is fatal in approximately 30% of those who show symptoms, leaving half of the survivors with permanent brain damage.
In the U.S., Valneva markets and distributes IXIARO® directly to the military and private travel market.
IXIARO / JESPECT® is licensed for adults in Australia, New Zealand, Europe, Canada, Switzerland, Hong Kong, Singapore, Israel, Norway, Liechtenstein, Iceland, Singapore, Japan, the United Kingdom, and the Republic of Korea. In all other licensed territories, IXIARO/JESPECT is indicated for use by adults aged 18 years or older.