Search API
The U.K. Health Security Agency (UKHSA) recently announced signing an advance purchase agreement with CSL Seqirus, who will be on standby to produce over 100 million influenza pandemic vaccines if or when they are needed.
UKHSA and its predecessor organizations have had similar agreements, but this is the first time the manufacturing process will be based entirely in the U.K., giving better security of access if global demand ever outweighs supply.
These vaccines will be produced at CSL Seqirus's existing manufacturing plant in Liverpool if a pandemic is declared by the World Health Organization (WHO).
Pandemic influenza is not the same as seasonal influenza or avian influenza.
Seasonal influenza circulates every year and is passed between people. Avian influenza, meanwhile, cannot be passed from human to human.
Although influenza pandemics are highly unpredictable in duration and severity, historical events show they can occur anytime.
Four influenza pandemics have occurred over the past 100 years, arising in 1918, 1957, 1968, and 2009.
The 1918 pandemic was responsible for over 50 million deaths worldwide, reports the UKHSA.
Professor Dame Jenny Harries, Chief Executive of UKHSA, commented in a press release on September 26, 2023, "We have seen from past pandemic events that access to effective vaccines is vital to help save lives and minimize disruption to our lives and livelihoods."
"This agreement represents a major step forward in our preparedness against future influenza pandemics."
CSL Seqirus is a global leader in pandemic influenza preparedness and annual flu shot production.
On July 17, 2023, CSL Seqirus announced it is positioned to supply over 55 million doses of QUADRIVALENT influenza vaccines (FLUCELVAX®, FLUAD®, AFLURIA®) to U.S. healthcare providers for the 2023-2024 flu season.
And has produced Audenz™, a monovalent, adjuvanted, cell-based inactivated subunit vaccine designed to protect people from disease caused by the influenza A virus H5N1 subtype in the event of avian influenza (bird flu) pandemics.

The New Hampshire Department of Health and Human Services, Division of Public Health Services (DPHS) recently announced that the risk for both mosquito- and tick-transmitted infections remains high and urged everyone to prevent bites.
This is essential advice since no preventive vaccines or anti-virus medications target Jamestown Canyon Virus (JCV) or Powassan Virus (POWV) diseases.
During the summer of 2023, fourteen batches of mosquitos around the state tested positive for JCV.
On September 25, 2023, DPHS confirmed a human JCV infection in an adult from Hillsborough County.
New Hampshire has reported a total of 13 human cases of JCV since 2018.
People infected with JCV can develop more serious central nervous system diseases, including meningitis or encephalitis.
About half of patients reported with JCV disease are hospitalized.
The good news is deaths associated with JCV infection are rare, according to the U.S. CDC.
Other infections transmitted by mosquitos in New Hampshire include Eastern Equine Encephalitis and West Nile Virus, which also present with symptoms similar to JCV.
The CDC has reported four states have confirmed JVC cases this year.
In addition, DPHS reported two cases of POWV in September, one in an adult in Rockingham County and another in a child from Carroll County.
New Hampshire has identified 8 cases of POWV since 2013, when the disease was first detected in humans.
During 2023, nine states have reported POWV cases.
Approximately half of the people who survive severe disease have long-term health problems such as recurring headaches, loss of muscle mass and strength, and memory problems.
And about 10% of people with severe POWV disease die.
“Mosquitoes will be with us until the first hard frost, and ticks remain active as long as there is no snow cover and temperatures remain above freezing,” commented Ryan Tannian, Chief of the DPHS Bureau of Infectious Disease Control, in a press release.
“Preventing the bites that cause illnesses transmitted by mosquitoes and ticks is a key factor in reducing the risk for illness.”
For national data on JCV and POWV, visit https://www.cdc.gov/jamestown-canyon/statistics/current-season-data.html and https://www.cdc.gov/powassan/statistics-data/current-season-data.html.
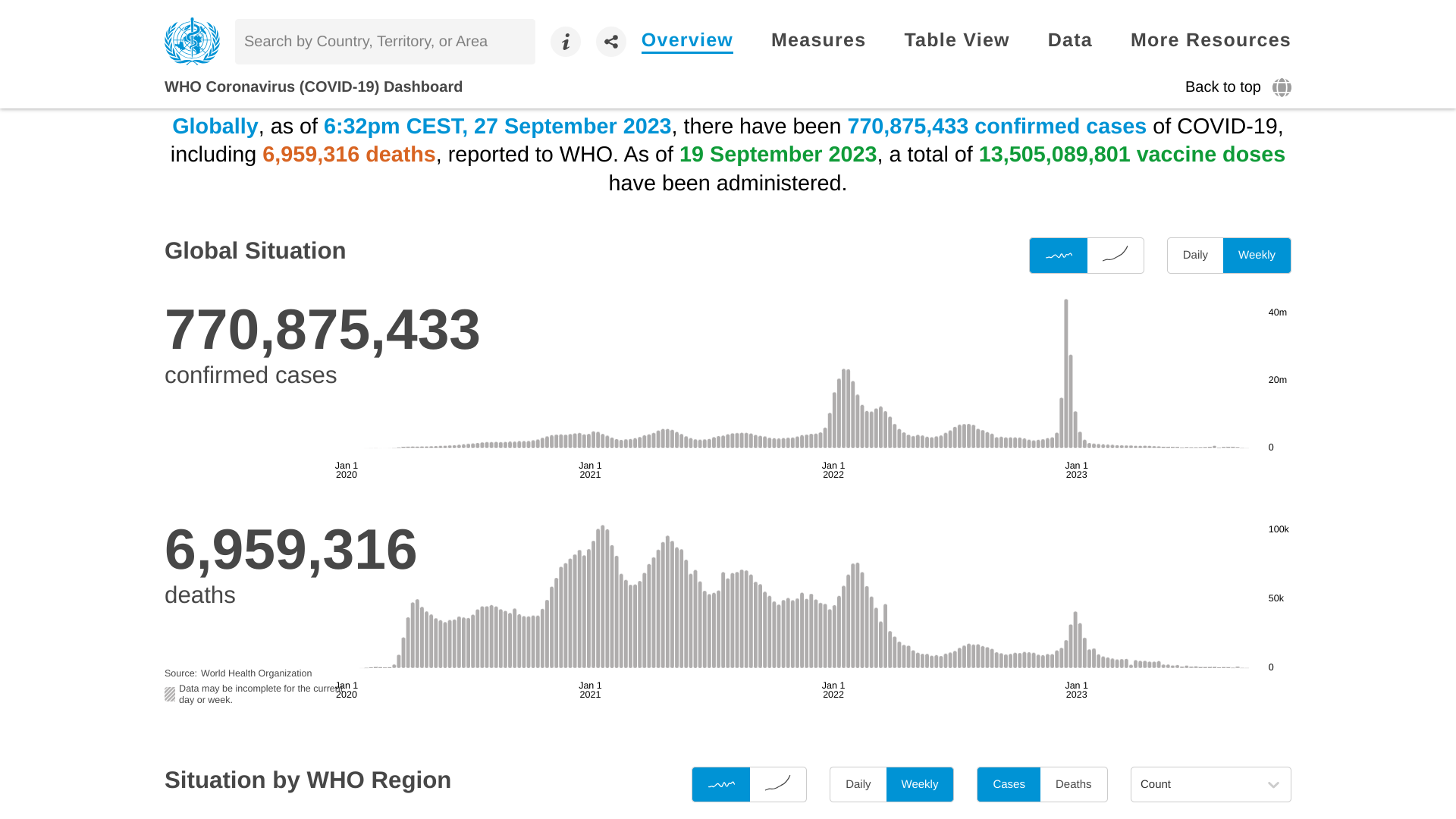
According to recent World Health Organization (WHO) data, COVID-19 cases and related deaths continue to decrease.
As of September 18, 2023, the WHO's data dashboard indicates a global 54.93% decrease in weekly COVID-19 cases and 33.85% decrease in deaths.
These positive trends have been reported since January 2023.
The WHO stated on September 12, 2023, that some regions and countries continue to see increases in cases and deaths; in others, they are declining.
While the emergency phase of the COVID-19 pandemic is over, countries should continue to work towards vaccinating at least 70% of their populations, prioritizing 100% of health workers and the most vulnerable groups, says the WHO (Edition 159).
As of September 19, 2023, a total of 13,505,089,801 COVID-19 vaccine doses have been administered during the pandemic, according to the WHO.
Since 2020, about 50 different COVID-19 vaccines have been deployed during the pandemic. With the recent U.S. FDA and CDC approvals, updated COVID-19 vaccines are now available in the United States.
According to numerous media reports, these innovative vaccines have faced availability constraints.
This was not the experience most U.S. residents witnessed during the early days of the pandemic, thanks in part to General Gus Perna, the four-star general of logistics who was the Chief Operations Officer of Operation Warp Speed.
As General Perna explained in this recent video, knowing your purpose is a key to success for most business, health, and life challenges.
Note: This article was updated on October 1, 2023, to include reference link.
Gritstone bio, Inc. today announced that it was awarded a contract by the U.S. Biomedical Advanced Research and Development Authority (BARDA) to conduct a Phase 2b comparative study evaluating Gritstone's self-amplifying mRNA (samRNA) CORAL vaccine candidate containing Spike plus other viral targets to protect against COVID-19.
Gritstone's approach seeks to generate a therapeutic immune response by leveraging insights into the immune system's ability to recognize and destroy diseased cells by targeting select antigens.
The agreement, valued at up to $433 million, was awarded as part of 'Project NextGen,' an initiative by the U.S. Department of Health and Human Services (HHS).
Under the HHS contract, Gritstone will conduct a 10,000-participant, randomized Phase 2b double-blinded study in the United States in collaboration with the COVID-19 Prevention Network.
Andrew Allen, M.D., Ph.D., Co-founder, President, and Chief Executive Officer of Gritstone bio, commented in a press release on September 27, 2023, "First-generation COVID-19 vaccines provided great utility during the height of the pandemic but are limited in breadth and durability of clinical protection."
"CORAL was designed to address these limitations by inducing durable neutralizing antibody and T cell-based immunity against current and future SARS-CoV-2 (betacoronavirus) variants."
"We are excited about this opportunity to work alongside BARDA and look forward to initiating the Phase 2b study (CORAL-BARDA) in the first quarter of 2024."
An introduction video is posted at this Gritstone About Us link.
This project has been funded with Federal funds from the Department of HHS, Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority, under Contract No. 75A50123C00062.
In August 2023, HHS awarded more than $1.4 billion for Project NextGen through the Administration for Strategic Preparedness and Response.
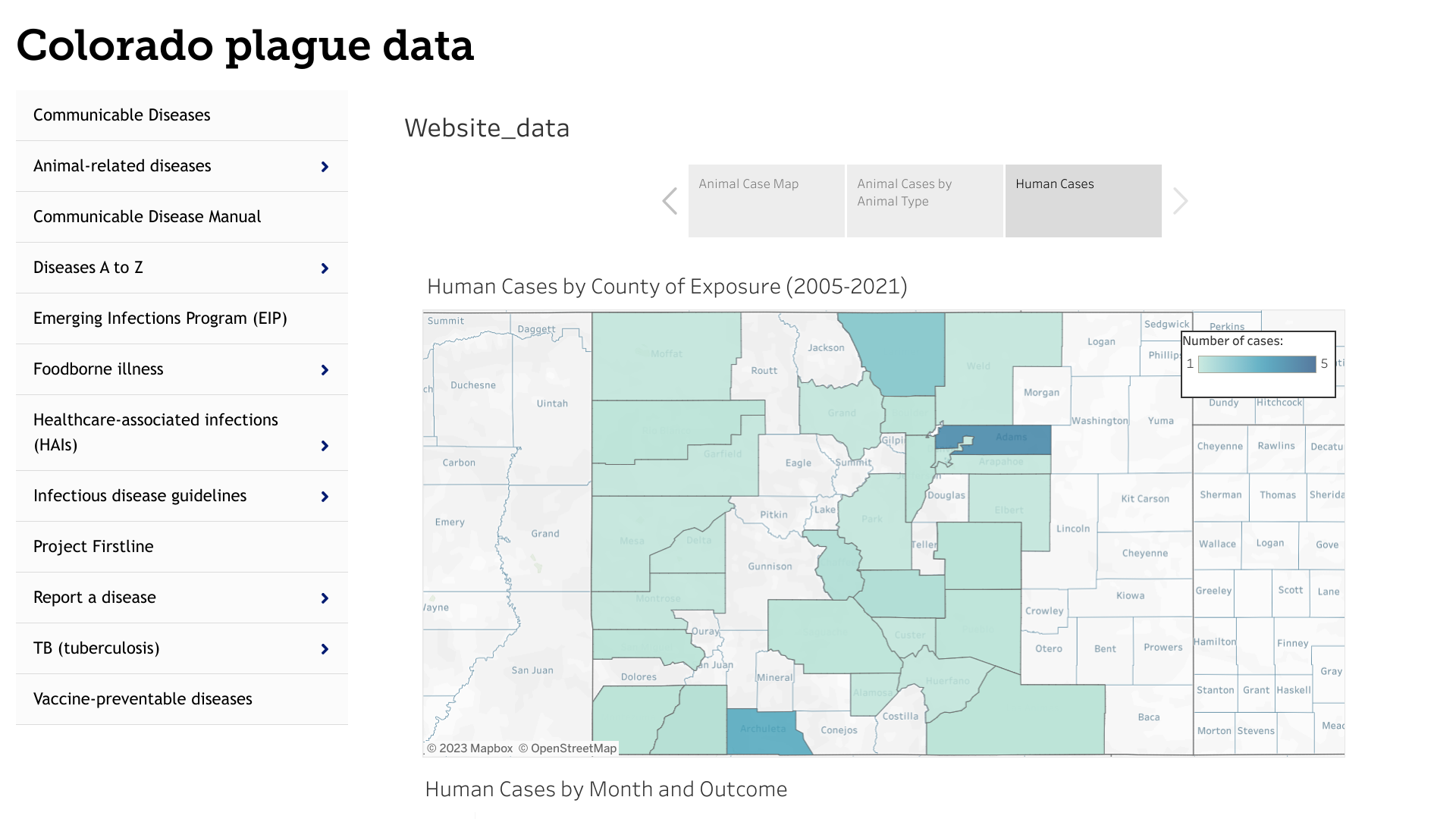
Recent testing has confirmed a case of plague associated with the death of an Archuleta County resident.
The Colorado Department of Public Health and Environment and San Juan Basin Public Health (SJBPH) announced on September 25, 2023, that they are investigating this plague case.
Most human cases are acquired directly from fleas, and pets with plague can transmit the illness to humans. Over 80% of United States plague cases have been the bubonic form, according to the Centers for Disease Control and Prevention (CDC).
Plague (Yersinia pestis) is caused by bacteria that can be transmitted to humans by the bites of infected fleas or by direct contact with infected animals. Symptoms include the sudden onset of high fever and/or swollen lymph nodes, according to SJBPH.
Tiffany Switzer, Interim Executive Director of SJBPH, commented in a press release, "Plague is frequently detected in rock squirrels, prairie dogs, wood rats, and other species of ground squirrels and chipmunks."
The Colorado previously reported a plague case in Montezuma County in June 2023.
The risk to the general public is low, says SJBPH.
Most human cases in the U.S. are reported in two regions: Northern New Mexico, northern Arizona, and southern Colorado; and California, southern Oregon, and far western Nevada.
SJBPH stresses the importance of controlling wildlife and fleas around homes.
Furthermore, see a healthcare provider if you become ill with a high fever and/or swollen lymph nodes, as plague is a treatable illness.
And contact a veterinarian if your pet becomes ill with a high fever and/or an abscess (open sore) or swollen lymph nodes.
Plague was first introduced into the United States in 1900 by rat–infested steamships that had sailed from affected areas.
The last urban plague epidemic in the United States occurred in Los Angeles from 1924 through 1925.
New plague vaccine candidates are developing but are not expected to be commercially available immediately.
However, researchers from Tel Aviv University and the Israel Institute for Biological Research's mRNA-based, lipid nanoparticle vaccine were effective against a lethal bacteria.
Published on March 8, 2023, a mice study demonstrated that all vaccinated animals were fully protected against the bacteria that causes the plague.
To learn more about the symptoms, treatments, and other information for plague, visit https://sjbpublichealth.org/183/Communicable-Disease. Information is also available from the Colorado Department of Public Health and Environment at https://cdphe.colorado.gov/animal-related- diseases/plague.
The Minnesota Health Department today confirmed that Beyfortus™ (Nirsevimab) is available to order via the Minnesota Vaccine for Children (MnVFC).
Beyfortus is the first U.S. FDA-approved extended half-life monoclonal antibody offering passive immunization to infants to prevent lower respiratory tract infections caused by the respiratory syncytial virus (RSV).
Even though Beyfortus is not a vaccine, the VFC program voted to include it in the VFC available vaccines to help ensure that all MnVFC-eligible children can access it at no cost.
Many Minnesota hospital pharmacies are enrolled in the MnVFC program because they manage vaccines for their co-located clinics.
These hospitals can provide inpatient Beyfortus as part of this program. Still, they may need to adjust their practices to include monitoring inpatient unit refrigerator temperatures and documenting MnVFC eligibility in their records.
Nationwide, Beyfortus is part of the U.S. Vaccines For Children program and will be available for the 23-24 RSV season.
The U.S. CDC says administration will start in early October 2023.
Note that babies born between April and September will receive doses early in fall 2023, in addition to babies born during the RSV season, which runs into 2024.

A company developing polysaccharide conjugate vaccines against serious bacterial threats announced today it received a grant award of up to $3 million for developing a Group B Streptococcus (GBS) vaccine.
The U.S. National Institute of Allergy and Infectious Diseases issued the grant to Omniose, whose research operations were established at BioGenerator Labs in St. Louis, MO. The company was previously known as VaxNewMo.
Timothy Cooke, CEO, commented in a press release on September 27, 2023, “GBS infections are an important health problem globally, and there is a broad consensus for a vaccine solution.”
Omniose aims to develop a GBS vaccine with the highest efficacy using a vastly simplified production process.
The Omniose platform uses synthetic biology with enzymatic rather than chemical methods to produce GBS polysaccharide conjugate vaccines.
The one-step enzymatic process occurs in a single re-engineered E. coli cell and retains 100% of sialic acid residues on the polysaccharide for each capsular polysaccharide conjugate.
Retention of sialic acid residues has been shown to be critical for eliciting optimal functional antibody responses towards certain GBS serotypes post-vaccination.
Conventional chemical methods are complex and lead to reduced levels of sialic acid residues through oxidation, which can have a negative impact on protection, says the company.
Group B Streptococcus is a leading cause of invasive bacterial infections in neonates and older adults in the U.S. It causes an estimated 90,000 infant deaths and 46,000 stillbirths worldwide, according to the U.S. CDC. There are currently no vaccines to prevent GBS.

The September KFF COVID-19 Vaccine Monitor recently asked the public about their intention to get an influenza vaccine, new respiratory syncytial virus (RSV) vaccines, and the updated COVID-19 vaccine during the 2023-2024 respiratory season.
A “tripledemic” scenario in which all three viruses peak together could impact millions of people simultaneously.
According to the KFF poll announced on September 27, 2023, most adults (58%), including three-quarters of adults 65 and older, say they will get a flu shot this year.
And about half of adults say they either will “definitely get” (23%) or “probably get” (23%) the new vaccine for COVID-19 that was approved on September 12.
In addition, 58% of adults 60 and older say they will either “definitely get” or “probably get” the new RSV vaccine recommended for their age group.
The U.S. Centers for Disease Control and Prevention (CDC) recently recommended that most people get vaccinated this year.
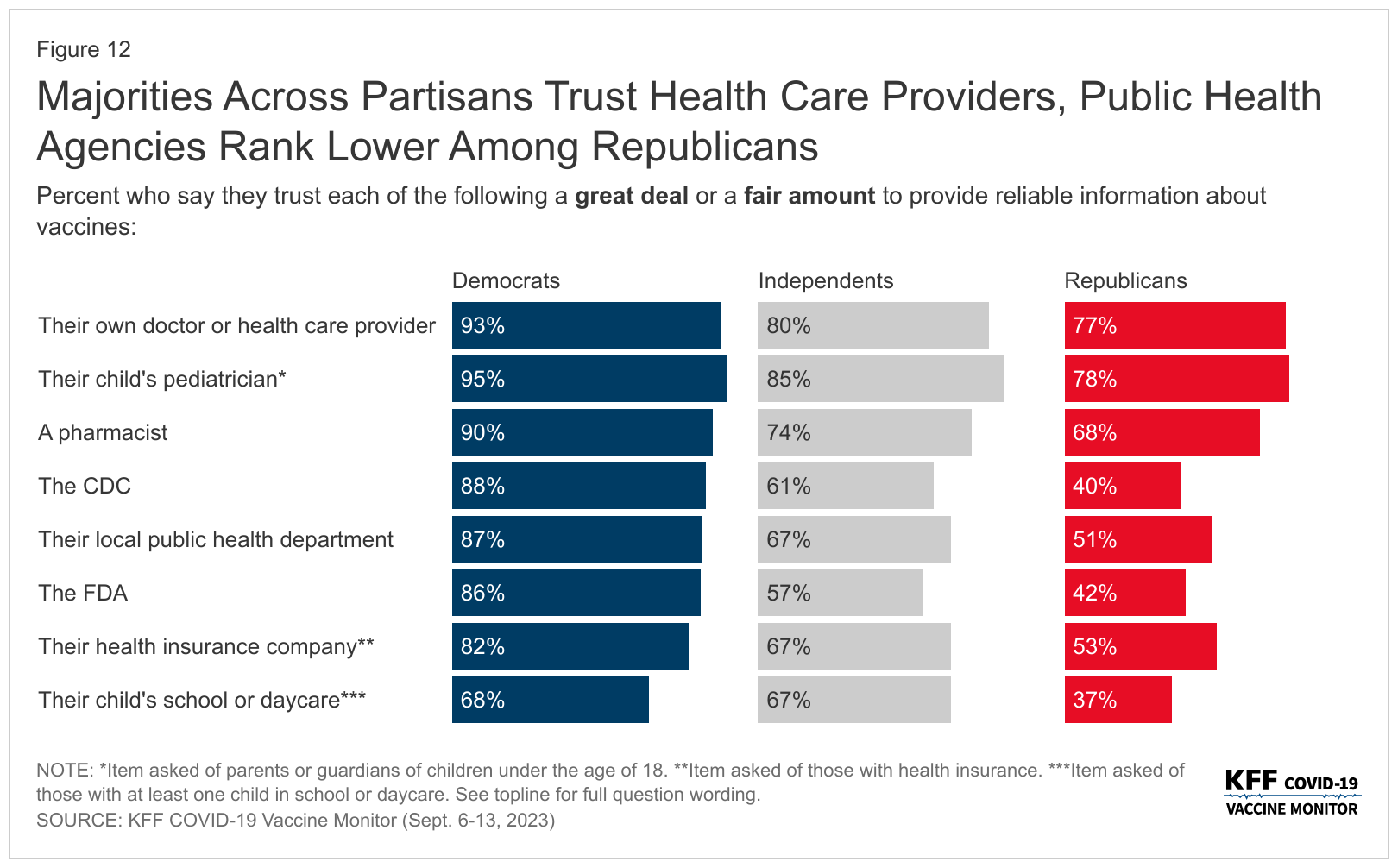
From a trust perspective, this poll offered good news for healthcare providers.
Healthcare providers were ranked as the most trusted sources of information about vaccines again, as most adults (68%) say they usually keep up-to-date with the vaccines their provider recommends.
Unfortunately, government sources of vaccine information like the CDC, local public health departments, and the U.S. FDA fare much lower.
The complete KFF poll results are linked here.
This article's headline was updated on Sept. 28, 2023.