Search API
In a new study published by BMC Public Health, researchers investigated the effect of zoster vaccination on dementia risk in an extensive UK population-based data set.
On October 2, 2023, these researchers disclosed an inverse association between zoster vaccination and dementia outcome in a fully adjusted model (HR 0.78, 95% CI: 0.77–0.79).
For Alzheimer's disease, the effect size is smaller (HR 0.91, 95% CI: 0.89–0.92); however, this result is likely only significant because of the large sample size involved.
To investigate if the result seen was exclusive to HZ vaccination, we also explored the effect of influenza vaccine and dementia/Alzheimer's disease.
They found a slight decreased hazard risk with HR of 0.96 (95% CI: 0.94–0.97) for dementia and HR of 1.10 (95% CI: 1.07–1.12) for Alzheimer's disease.
It has been estimated that the number of people with dementia could increase from 57 million cases in 2019 to 152 (130.8–175.9) million cases worldwide in 2050.
These researchers wrote, 'Several population-based studies have suggested a link between herpes zoster infection or vaccine against shingles and dementia. However, other population-based studies did not show any association between zoster infection and dementia risk.'
Globally, there are several approved shingles (herpes zoster) vaccines.
In the United States, GlaxoSmithKline plc Shingrix® is generally available at health clinics and pharmacies and has been reported up to 90% effective against shingles infection in various clinical trials.

Malaria vaccines have been in development since the 1960s, with substantial progress in the last decade, says the World Health Organization (WHO). Today, the WHO announced it has recommended a second vaccine, R21/Matrix-M, for preventing malaria in children.
As of October 2023, the R21 vaccine is the second malaria vaccine recommended by WHO, following the Mosquirix™ (RTS,S/AS01) vaccine, which received a WHO recommendation in 2021.
Demand for malaria vaccines is unprecedented in 2023. However, the available supply of RTS,S is limited.
The addition of R21 to the WHO's recommended malaria vaccine list is expected to result in sufficient vaccine supply in various countries.
The following steps for R21/Matrix-M, include completing the ongoing WHO prequalification and enabling international vaccine procurement for a broader rollout in malaria outbreak countries.
The WHO's Director-General endorsed the recommendation following its regular biannual meeting on 25-29 September 2023.
"As a malaria researcher, I used to dream of the day we would have a safe and effective vaccine against malaria. Now we have two," commented Dr Tedros Adhanom Ghebreyesus, WHO Director-General, in a press release on October 2, 2023.
"Demand for the RTS,S vaccine far exceeds supply, so this second vaccine is a vital additional tool to protect more children faster and to bring us closer to our vision of a malaria-free future."
The R21 vaccine has been shown to reduce symptomatic malaria cases by 75% during the 12 months following a 3-dose series. A fourth dose given a year after the third maintained efficacy.
This efficacy is similar to that demonstrated when RTS,S is given seasonally.
The WHO says that at US $2 – US $4 per dose, the cost-effectiveness of the R21 vaccine would be comparable with other recommended malaria interventions and other childhood vaccines.
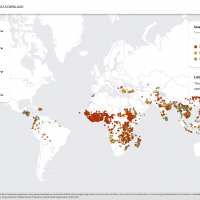
Malaria is a disease caused by four species of protozoan parasites of the genus Plasmodium and is transmitted to people by Anopheles mosquitoes.
According to the WHO's recent World Malaria Report, the global number of malaria cases reached about 240 million, with over 600,000 related fatalities.
As of October 2023, neither malaria vaccine is available in the United States.
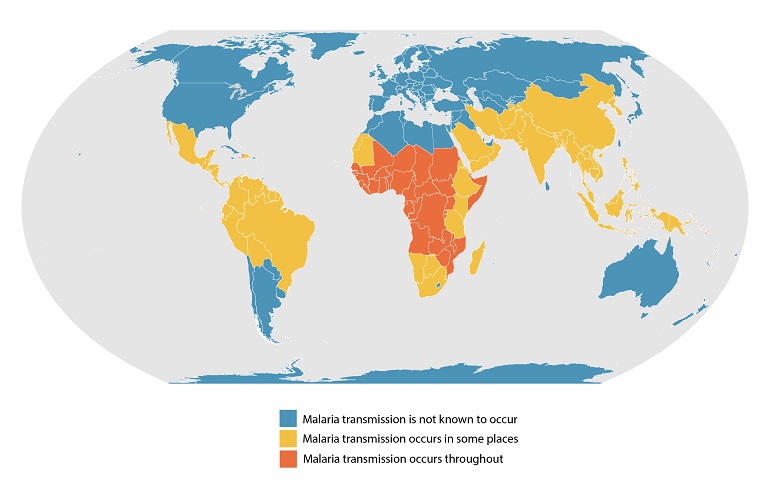
Dengue outbreaks continue to pose significant public health burdens in endemic countries and, unfortunately, may continue to increase in incidence as disease-carrying mosquitoes expand geographically, according to the World Health Organization (WHO).
To reduce the severity of dengue outbreaks, the WHO today announced the live-attenuated quadrivalent dengue vaccine Qdenga® (TAK-003) developed by Takeda has been confirmed to demonstrate efficacy against all four serotypes of the dengue virus in baseline seropositive children (4-16 years) in endemic countries.
And against serotypes 1 and 2 in baseline seronegative children.
The WHO's Strategic Advisory Group of Experts on Immunization (SAGE) on Immunization recommended on October 2, 2023, that Qdenga be considered for introduction in settings with high dengue disease burden and high transmission intensity to maximize the public health impact and minimize any potential risk in seronegative persons.
The SAGE now recommends introducing Qdenga to children aged 6 to 16.
The vaccine should be introduced within this age range about 1-2 years before the age-specific peak incidence of dengue-related hospitalizations.
Qdenga should be administered in a 2-dose schedule with a 3-month interval between doses.
The WHO will consider the SAGE recommendation and update its paper on dengue vaccines to include final guidance on using Qdenga in public vaccination programs.
"The global impact of dengue cannot be overlooked as the incidence continues to rise. This week, the WHO's SAGE provided important recommendations for the use of QDENGA in preventing dengue," commented Gary Dubin, M.D., president of the Global Vaccine Business Unit at Takeda, in a press release on October 3, 2023.
While approved for use in Brazil and various European countries, Qdenga is unavailable in the U.S.
On July 11, 2023, Takeda announced that the Company has voluntarily withdrawn Qdenga's U.S. Biologics License Application following discussions with the U.S. Food and Drug Administration on aspects of data collection.
However, the Dengvaxia® vaccine is both FDA and WHO-recommended where appropriate.
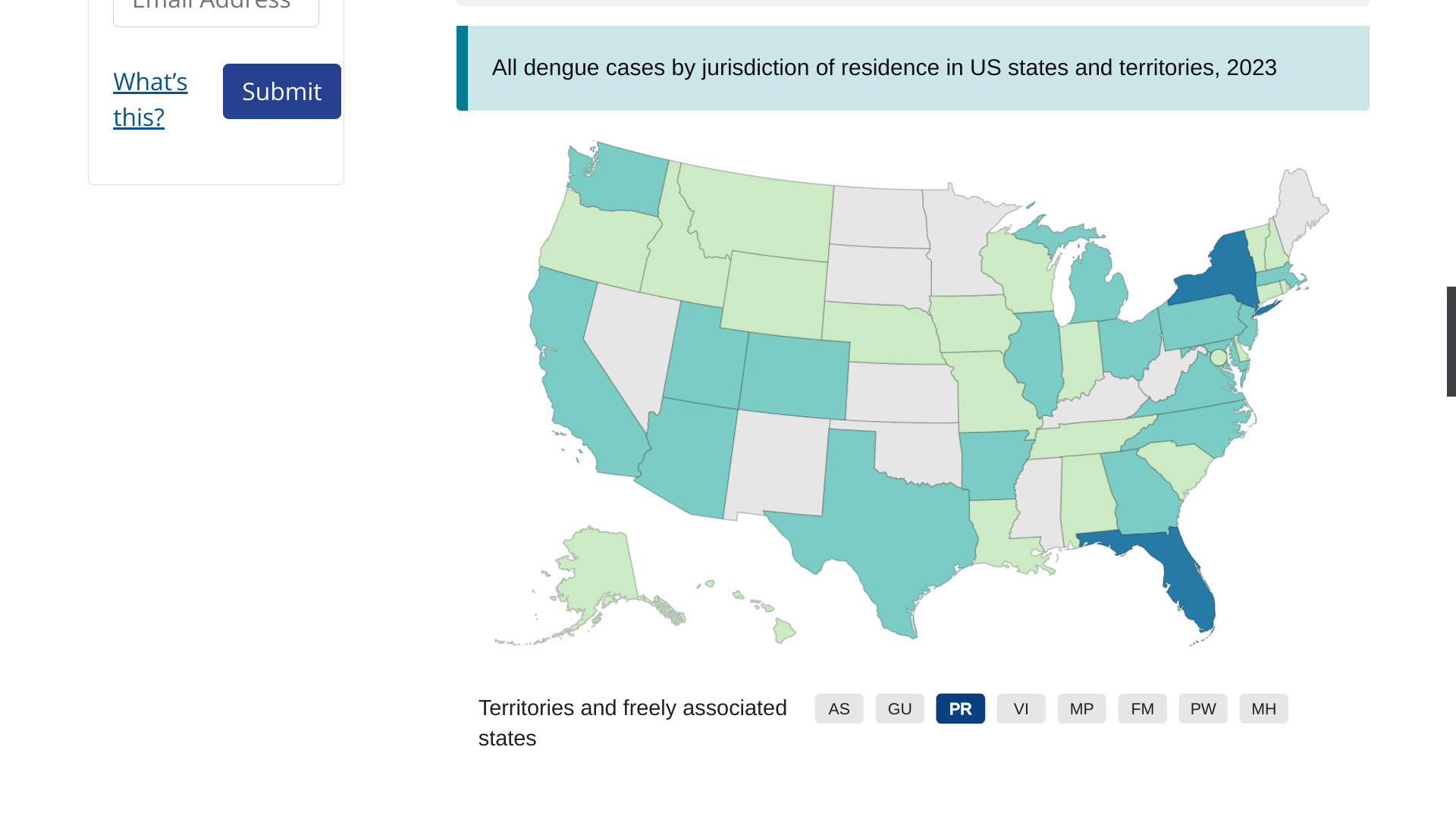
As of September 13, 2023, 44 U.S. jurisdictions had reported about 997 dengue cases this year. Throughout the summer of 2023, dengue outbreaks have been reported in southern Florida and Puerto Rica.
Note: This article was updated on October 3, 2023, to include the Company's press release.
Without an approved Alzheimer's disease vaccine available, several bipolar disorder (BD) candidates are following U.S. Food and Drug Administration (FDA) policies to accelerate clinical research.
In the U.S., about 2.8% of the population is estimated to be affected by BD during the past year, says the U.S. NIH.
For example, Alzamend Neuro, Inc. today announced receipt of a “Study May Proceed” letter from the U.S. FDA for the initiation of study AL001-BD01, a Phase IIA clinical study of AL001 for BD type 1.
AL001 is a novel lithium-delivery system that has the potential to deliver benefits of marketed lithium salts while mitigating or avoiding currently experienced toxicities associated with lithium, a chemical entity well known for efficacy in BD type 1.
Alzamend’s goal is to revive the utility of lithium treatment by importantly improving the benefit-to-risk relationship of lithium treatment in clinical practice.
“Lithium was the first mood stabilizer approved by the FDA and is still a first-line treatment option for BD type 1,” said Stephan Jackman, Chief Executive Officer of Alzamend, in a press release on Octobe 2, 2023.
“If we are able to develop a next-generation lithium product (AL001) that would not routinely require therapeutic drug monitoring, it would constitute a major improvement over current lithium-based treatments and positively impact the seven million Americans afflicted with BD."
"We are advancing the process and expect that the first patient will be dosed in the first quarter of 2024"
Based on the favorable safety profile observed in the recently completed study and extensive safety data on the drug’s constituent components, the AL001 development program may qualify for a Section 505(b)(2) New Drug Application pathway for FDA approval, which is available to new formulations of an approved drug.
BD is a mood disorder characterized by periods of depression and periods of abnormally elevated happiness.
The condition is classified as BD Type 1 if there has been at least one manic episode, with or without depressive episodes.
And can be classified as BD Type 2 if there has been at least one hypomanic episode (but no full manic episodes) and one major depressive episode.
As of October 2023, there are several Alzheimer's disease vaccines and therapies conducting clinical trials which are seeking participants.

According to Reuters reporting, about 1.8 million people in the United States received an updated mRNA COVID-19 vaccine during the week ended September 22, 2023, according to data compiled by IQVIA Holdings Inc.
Moderna Inc. and Pfizer, Inc.'s newly approved vaccines are meeting pent-up consumer demand who are preparing for the forthcoming respiratory disease season in the U.S.
These mRNA vaccines are generally available in U.S.-based pharmacies and health clinics.
Pharmacies such as Walgreens have recently updated their vaccine appointment scheduling capabilities and related information.
As of October 2, 2023, U.S. agencies have not approved Novavax Inc.'s protein-based COVID-19 Vaccine Adjuvanted (Nuvaxovid™).
These innovative vaccines aspire to prevent the fading COVID-19 pandemic from further impacting the global society.

Recent research assessed whether the off-label use of meningococcal group B vaccines (MBV) was associated with subsequent lower gonorrhea prevalence.
A Research Letter published by the JAMA Network Infectious Diseases on August 31, 2023, concluded the outer membrane vesicle OMV-based MBV was 47% (95% CI, 13%-68%) effective in preventing gonorrhea among recipients aged 18 to 29 years.
These results are consistent with other study findings that OMV-based vaccines offered 31% (95% CI 21-39) protection against gonorrhea.
Two meningococcal vaccines are available in the U.S.: MenB-4C (OMV-based) and MenB-FHbp.
This study's finding is good news since gonorrhea infection rates increased by about 4%, reaching 710,151 in 2022.
As of September 30, 2023, the U.S. Food and Drug Administration (FDA) and the European Medicines Agency have not approved vaccines for preventing gonorrhea infection.
However, the FDA recently granted Fast Track designation for GSK's Neisseria gonorrhea investigational vaccine (NgG) candidate.
GSK announced on June 27, 2023, that Phase II of an ongoing study aims to demonstrate Proof of Concept by assessing the NgG vaccine's efficacy in healthy adults at risk of gonorrhea.
This study started in November 2022 in the U.S., UK, France, Germany, Spain, Brazil, Philippines, and South Africa.
According to the U.S. Centers for Disease Control and Prevention, gonorrhea is now the second most common sexually transmitted infection (STI) in the U.S. Most women infected with gonorrhea do not have any symptoms.
Other STI vaccine news (herpes, syphilis, HPV, mpox) is posted at this link.

The World Health Organization (WHO) today announced its vaccine recommendations for the 2024 influenza season in the southern hemisphere.
For countries in tropical and subtropical regions, the WHO recommendations for influenza vaccine composition (NH or SH) are available on the WHO Global Influenza Programme website as of September 29, 2023.
The WHO also recommended removing the influenza B/Yamagata/16/88 lineage component, which has not been detected since early 2020.
While influenza vaccines are safe and effective, manufacturing and using inactivated and live attenuated vaccines containing B/Yamagata lineage viruses pose a theoretical risk of reintroducing the B/Yamagata lineage virus into the population.
This risk can be mitigated by removing B/Yamagata lineage viruses from the vaccines.
Therefore, the WHO Influenza Vaccine Composition Advisory Committee believes that including a B/Yamagata antigen as a component of influenza vaccines is no longer warranted, and every effort should be made to exclude it as soon as possible, wrote the WHO.
Furthermore, when the WHO recommends viruses for inclusion in influenza vaccines twice each year, it is up to national or regional authorities to approve the composition and formulation of vaccines used in each country.
These new WHO recommendations do not impact the 2023-2024 flu shots currently offered in the United States.
As of September 16, 2023, 83.59 million influenza vaccine doses had been distributed in the U.S. Annual flu shots, which the U.S. CDC recommends for most people, are readily available at most pharmacies in the U.S.
The Texas Department of State Health Services (DSHS) recently confirmed October 1, 2023, is Influenza Awareness Day, and everyone should prepare themselves to combat this respiratory virus.
DSHS says each flu season in Texas has its own characteristics.
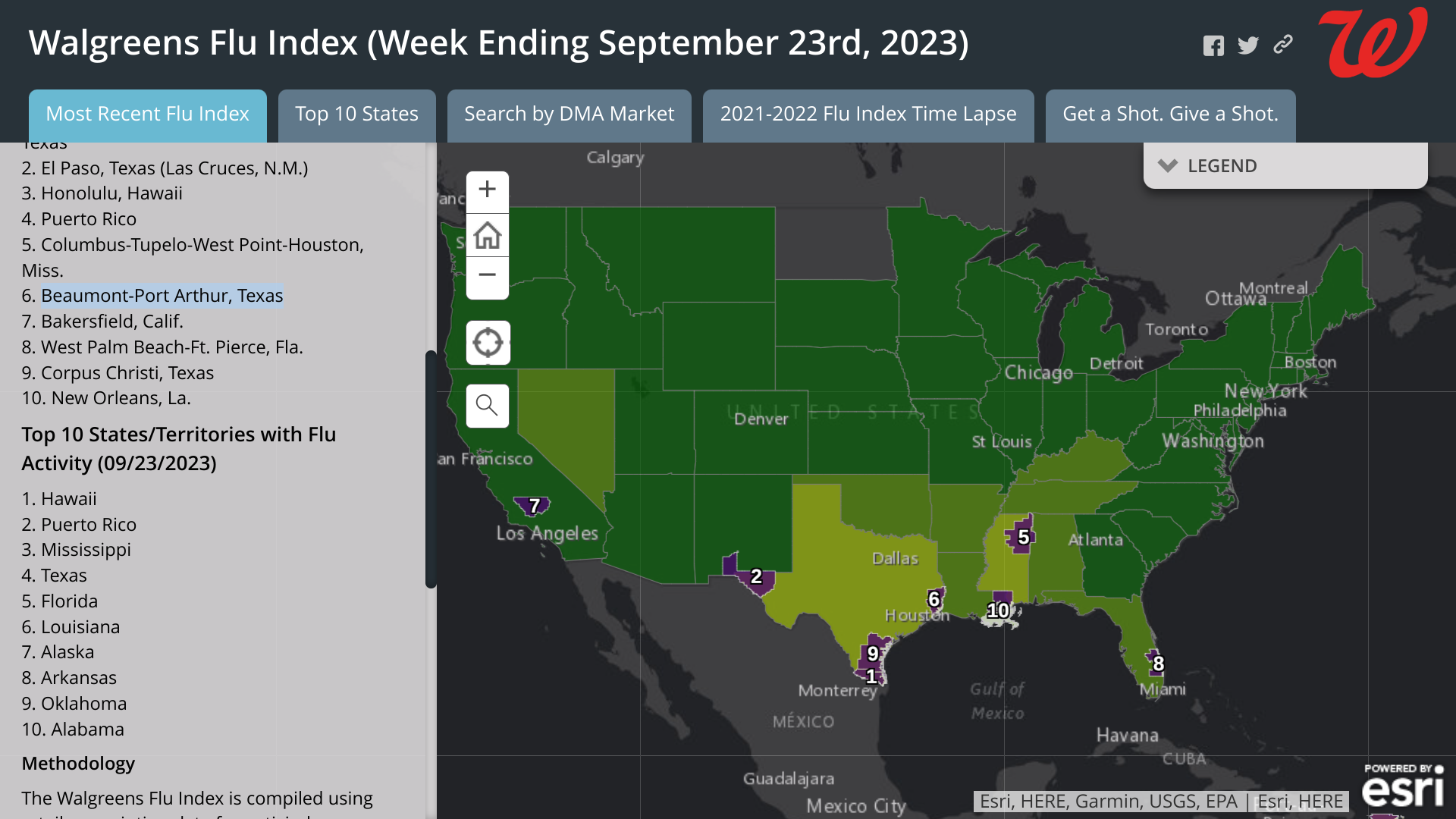
According to the latest Walgreens Flu Index, Texas is ranked #4 in overall flu cases, with Beaumont-Port Arthur and Corpus Christi in the top ten cities.
“Seasonal influenza presents a real public health threat to Texans, and immunization remains our best defense against serious flu illness,” said DSHS Commissioner Jennifer Shuford, MD, in a September 29, 2023 press release.
“Other actions can also help protect you and people close to you, like covering coughs and sneezes, washing your hands often, and staying home from work or school when you are sick.”
DSHS recommends Texans six months of age and older get the flu shot by the end of October, as immunization can make flu symptoms less severe and reduce flu-related deaths and hospitalizations.
The Centers for Disease Control and Prevention recently confirmed about 10,000 people die each year from influenza.
Influenza viruses are spread mainly by tiny droplets when people with flu talk, sneeze, or cough.
You can also contact your doctor, local health department, or pharmacy to learn where to get a flu shot, says DSHS.
Most Texas pharmacies enable online appointment scheduling and offer a selection of influenza vaccines.
For more information about influenza and how to protect against flu illness, visit dshs.texas.gov/influenza-flu.
To better address the evolving risk of type 2 circulating vaccine-derived poliovirus (cVDPV2), the Global Polio Eradication Initiative (GPEI) and partners have deployed the novel oral polio vaccine type 2 (nOPV2) to millions of people.
The nOPV2 vaccine has shown to be more genetically stable than older polio vaccines and less likely to be associated with the emergence of cVDPV2 in low immunity settings.
This means that nOPV2 has the potential to be a significant tool to help stop polio outbreaks, says the GPEI.
There have been over 30 countries reporting polio/poliovirus outbreaks in 2023.
The U.S. CDC's Advisory Committee on Immunization Practices presentation on October 19, 2022, confirmed that nOPV2 is more genetically stable and less likely to be associated with the emergence of cVDPV2 and can provide mucosal immunity to limit the virus's spreading among Inactivated polio vaccine (IPV)-vaccinated people.
According to the GPEI on September 27, 2023, nOPV2 has been deployed under the WHO's Emergency Use Listing procedure.
Approximately 750 million doses of nOPV2 have been administered across 35 countries. An additional 15 countries have met the requirements for nOPV2 use in the event of a poliovirus outbreak.
As of September 29, 2023, the United States has not approved the nOPV2 vaccine.
The CDC currently recommends that all children get vaccinated to protect against polio or poliomyelitis as part of the childhood vaccines.
The IPV is the only polio vaccine that has been given in the United States since 2000.