Search API
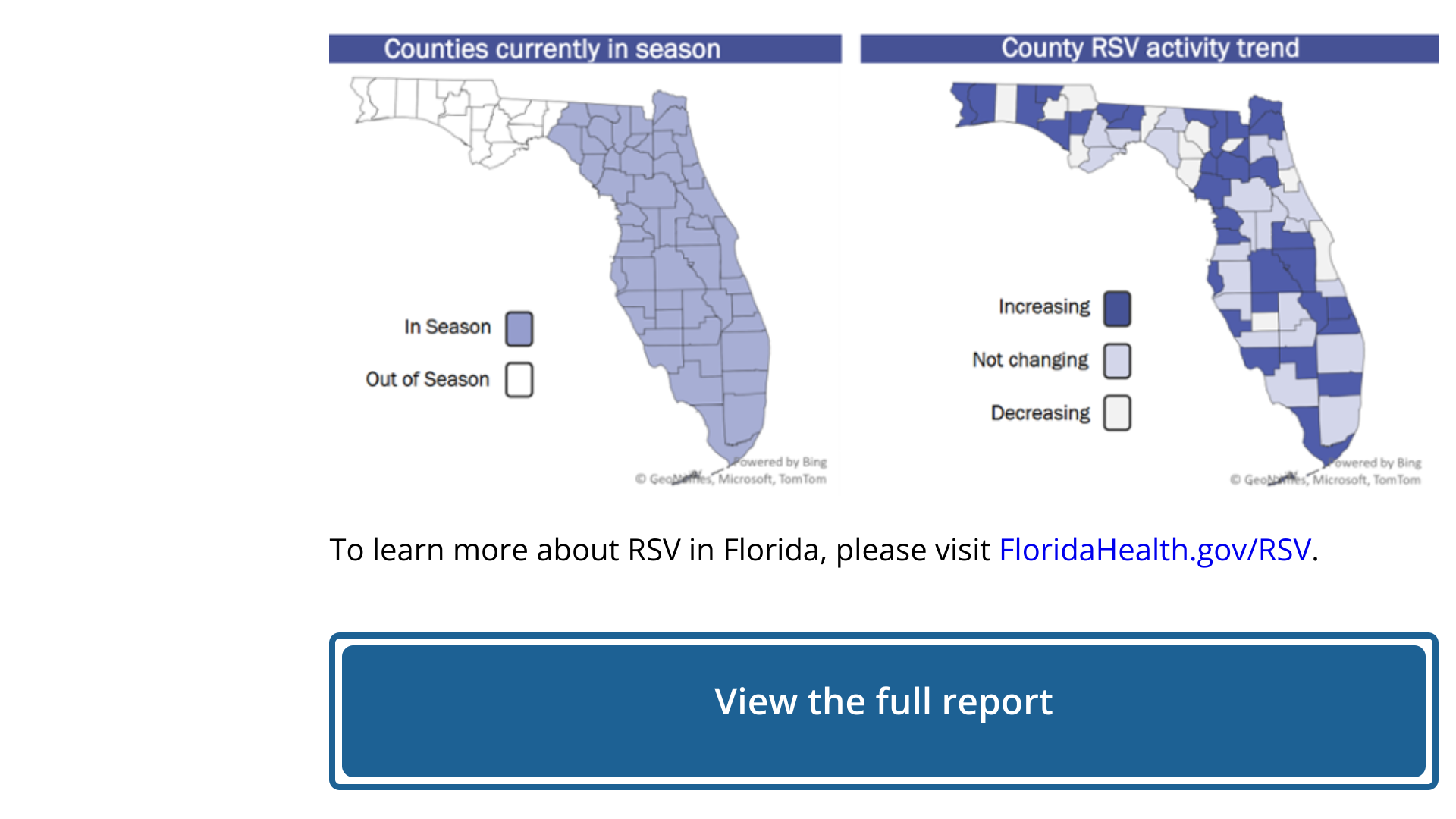
While the United States community is debating where and when the 2023-2024 flu season will arrive, the Walgreens Flu Index® ranks the top markets and states for flu activity, including Puerto Rico.
As of September 30, 2023, cities in the southern U.S. led the Top 10 Designated Market Areas with Flu Activity.
- Harlingen-Weslaco-Brownsville-McAllen, Texas
- Puerto Rico (San Juan)
- El Paso, Texas (Las Cruces, N.M.)
- Beaumont-Port Arthur, Texas
- New Orleans, La.
- Columbus-Tupelo-West Point-Houston, Miss.
- Honolulu, Hawaii
- Corpus Christi, Texas
- Houston, Texas
- Miami-Ft. Lauderdale, Fla.
From a more clinical perspective, the U.S. Centers for Disease Control and Prevention (CDC) today published its week #39 Influenza Surveillance Report.
As of October 6, 2023, seasonal flu rates last week were low nationally, with 444 (1%) positive specimens reported last week.
Additionally, 1,040 patients with laboratory-confirmed influenza were admitted to a hospital, an increased number since the previous CDC report.
And the U.S. National Center for Health Statistics mortality surveillance data distributed on October 5, 2023, confirmed that 11 additional influenza-related deaths were reported last week.
Of the flu-related deaths reported from October 2, 2022, to September 9, 2023, 9,697 (4%) listed influenza.
This data exceeded the average number of influenza-coded deaths (8,530) from 2015-16 through 2019-20.
Among 5,390 hospitalized adults with information on underlying medical conditions, 96.8% had at least one reported underlying medical condition; the most commonly reported were hypertension, cardiovascular disease, metabolic disorder, and obesity.
The CDC's new Director, Mandy K. Cohen, MD, MPH, recently answered questions and offered the most up-to-date information and common-sense solutions so you can protect yourself and your loved ones this respiratory season.
This week's updated flu shot availability news indicated that over 100 million influenza vaccines had been distributed to health clinics and pharmacies in the U.S.
Without preventative vaccines available for three well-known sexually transmitted diseases, the U.S. Centers for Disease Control and Prevention (CDC) recently took action by opening a docket to obtain comments on proposed guidelines for the use of doxycycline post-exposure prophylaxis (PEP) for prevention of bacterial sexually transmitted infections (STI).
Announced on October 2, 2023, the proposed guidelines for bacterial STI prevention include post-exposure prophylaxis with doxycycline (doxycycline PEP) because it has demonstrated benefit in reducing chlamydia, gonorrhea, and syphilis infections.
The incidence of STIs caused by Neisseria gonorrhoeae (causative agent of gonorrhea), Chlamydia trachomatis (causative agent of chlamydia), and Treponema pallidum (causative agent of syphilis) continues to increase in the U.S., says the CDC.
The proposed guidelines provide updated clinical guidance for healthcare providers to inform the use of doxycycline PEP for preventing bacterial STI infections.
Written comments must be received on or before November 16, 2023.
Doxycycline PEP, when offered, should be implemented in the context of a comprehensive sexual health approach, including risk reduction counseling, STI screening and treatment, recommended vaccination, and linkage to HIV pre-exposure prophylaxis (PrEP), HIV care, or other services, as appropriate.
The CDC has made available a pre-recorded informational presentation to provide information about the studies considered when developing the proposed guideline, explain the public comment process, and provide an overview of important monitoring for antibiotic use and antibiotic resistance that the agency will consider to address potential risks.
You may submit comments identified by Docket No. CDC–2023–0080 at Federal eRulemaking Portal: http://www.regulations.gov. Follow the instructions for submitting comments. Do not submit comments by email, as the CDC does not accept comments by email.

An independent committee of vaccine experts unanimously endorsed removing one influenza virus strain from future influenza vaccines.
On October 5, 2023, the Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted to eliminate the influenza B/Yamagata lineage viruses since it has not been detected for about three years.
The VRBPAC vote follows the World Health Organization's recent announcement that it endorsed eliminating the influenza B/Yamagata/16/88 lineage component from the 2024 Southern Hemisphere flu shot formulation.
Flu seasons in the Southern Hemisphere usually occur between April and September, while the Northern Hemisphere's is generally between October and May.
This change should not impact the effectiveness of flu shots.
A recent U.S. CDC report found the adjusted vaccine effectiveness against severe acute respiratory infection hospitalization associated with any influenza virus during the 2023 Southern Hemisphere flu season was 51.9% (95% Confidence Interval 39.2%–62.0%).
For the 2023-2024 flu season in the U.S., there should be ample supply of vaccines at health clinics and pharmacies. According to a report by the Global Healthy Living Foundation and IQVIA, about 60% of vaccinations during the 2022 flu season took place at pharmacies in the U.S.
Vaccine manufacturers have projected to supply as many as 156.2 million to 170 million doses of influenza vaccines for the 2023-2024 season.
As of September 23, 2023, the CDC says about 100 million doses had been distributed in the U.S. All influenza vaccines are quadrivalent for this flu season, such as FLUMIST® and FLUCELVAX®.

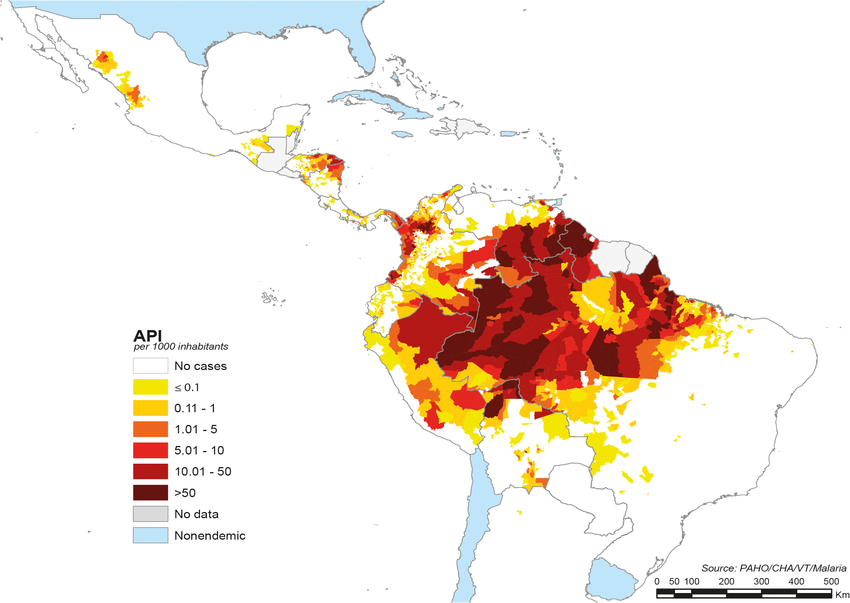
The Arkansas Department of Health (ADH) today announced it identified a case of locally acquired malaria in a resident who resides in Saline County and has no international travel history.
ADH stated in a press release on October 4, 2023, that this is the only known locally acquired case of malaria in Arkansas and the 11th in the United States in 2023
Earlier in 2023, the U.S. Centers for Disease Control and Prevention (CDC) confirmed seven locally acquired malaria infections in Florida, one in Texas, and one in Maryland.
On September 7, 2023, the Pan American Health Organization estimated that approximately 41 million people are living in areas where the risk of infection by malaria is considered moderate to high in 21 Latin American countries.
The CDC has issued various outbreak alerts for malaria-endemic countries, including Costa Rica.
According to the CDC, malaria is a vaccine-preventable disease caused by four species of protozoan parasites of the genus Plasmodium. These disease-carrying mosquitoes are found throughout the Americas.
There are two malaria vaccines in use in Africa in 2023. However, they are not available in the U.S.
While there is currently no cure for cat allergy, a prevalent, life-long condition, an innovative therapeutic vaccine for humans has begun clinical trials.
Angany Inc. today announced that it has received clearance from U.K.'s Medicines and Healthcare Products Regulatory Agency for the first clinical study to be conducted on its vaccine candidate ANG-101 to treat human allergy to cats.
ANG-101 is a therapeutic vaccine that provides a disruptive disease-modifying approach for treating cat allergy.
Derived from its proprietary eBioparticle-Potentiated Immunotherapy™ technology, ANG-101 active immunotherapeutic ingredient is a unique 140 nm enveloped bioparticle (eBioparticle™) that mimics a virus in shape and size with its surface covered with thousands of copies of cat major allergen Fel d 1.
This clinical study is a first-in-human, open-label, and single-site evaluation of the new vaccine's safety, allergenicity, and immunogenicity in adult patients allergic to cat dander.
This early-stage clinical trial will be conducted under the guidance of Professor Stephen Durham and Dr Guy Scadding, two leading clinical allergy experts from Imperial College London.
Professor Durham commented in a press release on October 5, 2023, "The potential treatment of cat allergy using an auto-adjuvanted vector builds upon its known ability to induce strong allergen-specific IgG antibody responses, as observed in animal models."
Unlike prophylactic vaccines, Angany’s therapeutic vaccines are a new generation of immunotherapy biologics used to treat established pathologies.
They are meant to restore or boost natural immune mechanisms and create sustainable immune protection and vigilance.
Human allergies to cats and dogs affect 10 to 20% of the world’s population, says the Asthma and Allergy Foundation of America.
When you have a pet allergy, you are not allergic to the pet’s hair, fur, or feathers but to the protein found in the pet’s dander, saliva, and urine.

Two world-class pharmaceutical companies announced an agreement for a potential first-in-class vaccine against extraintestinal pathogenic E. coli.
On October 3, 2023, France-based Sanofi confirmed it has agreed with Janssen Pharmaceuticals, Inc., a Johnson & Johnson company, to develop and commercialize a 9-valent vaccine candidate for extraintestinal pathogenic E. coli currently in Phase 3 study.
The agreement combines Janssen's robust science behind this potential first-in-class product, Sanofi's worldwide manufacturing footprint, and the recognized world-class expertise of these companies in launching innovative vaccines.
This is important news since Extraintestinal pathogenic E. coli is a leading cause of sepsis, particularly in older adults, with an approved vaccine available in 2023.
Sepsis is a life-threatening bloodstream infection accompanied by severe illness and widespread organ damage generated by the body's self-destructive response to the infection.
Thomas Triomphe, Executive Vice President, Vaccines, at Sanofi, commented in a press release, "E. coli is a significant cause of sepsis, mortality, and antimicrobial resistance in older adults, and the number of cases is rising as the population ages."
"In line with our commitment to design and deliver first- or best-in-class medicines and vaccines, this agreement with Janssen aims to positively impact public health by reducing hospitalization costs and the burden on health systems associated with ExPEC and help older adults around the world to live longer healthier lives."
The ongoing Phase 3 E.mbrace clinical trial is designed to evaluate the efficacy of the 9-valent extraintestinal pathogenic E. coli vaccine (ExPEC9V) compared to placebo in preventing invasive E. coli disease caused by ExPEC9V O-serotypes.
The study was started in 2021 by Janssen and continues to enroll patients. To learn more, visit https://classic.clinicaltrials.gov/ct2/show/NCT04899336.
Under the terms of the new agreement, both companies will co-fund current and future research and development costs.
Sanofi will pay USD 175M upfront to Janssen, followed by development and commercial milestones. A profit-share arrangement will exist in the U.S., EU4 (France, Germany, Italy, Spain), and the U.K. In the rest of the world, Janssen will receive tiered royalties and sales milestones.

Across both prepandemic and pandemic years, the respiratory syncytial virus (RSV) outbreaks in the United States began in Florida, the southeast, and later in the north and west regions.
Florida's RSV season is longer than the rest of the U.S. and has distinct regional patterns, says the U.S. Centers for Disease Control and Prevention (CDC).
The Florida Department of Health reported as of week #39, September 30, 2023, RSV activity was increasing with higher test positivity, hospital admissions, and emergency room rates, with three previous outbreaks but no current RSV outbreak.
Furthermore, NREVSS data show Florida's PCR positivity 3-week moving average was about 7.7% as of September 28, 2023.
Globally, the World Health Organization (WHO) Influenza Update N° 454 indicates RSV activity was found to be generally low, except in some parts of Western Australia and some Central and temperate South American countries.
Additional WHO and CDC RSV outbreak data are posted at Precision Vaccinations.
As of October 4, 2023, RSV vaccines and monoclonal antibody therapies are available in the U.S.