Search API
A glioblastoma vaccine therapy candidate has been granted Fast Track Designation (FTD) by the United States Food and Drug Administration (FDA).
Announced on October 12, 2023, MimiVax Inc.'s SurVaxM vaccine (immunotherapy) is being studied to treat newly diagnosed glioblastoma (nGBM).
Glioblastoma is a rare disease with great unmet medical need.
SurVaxM is a first-in-class, patented peptide mimic immunogen that targets survivin, a cell-survival protein present in 95% of glioblastomas and in many other cancers.
SurVaxM stimulates a patient’s immune response to control tumor growth and prevents disease recurrence. Because survivin is present in most cancers, SurVaxM could potentially have applicability in other cancers.
"The receipt of FTD affirms the importance of new clinical developments of novel therapies to improve the treatment and outcomes for patients with newly diagnosed glioblastoma," said Michael Ciesielski, CEO of MimiVax, in a press release.
"This designation is a key component in our journey to help patients with glioblastoma to live longer."
A randomized, blinded, placebo-controlled Phase 2b clinical trial of SurVaxM for nGBM (SURVIVE) [NCT05163080] is now recruiting at 11 cancer centers across the U.S.
Previously, positive Final Data from the previous Phase 2a Study of SurVaxM for nGBM, published in the Journal of Clinical Oncology, found that 51% of patients receiving SurVaxM survived at least two years, and 41% survived at least three years.
The median Overall Survival of 25.9 months with nGBM in this study is considerably higher than expected with standard therapy alone.

Throughout 2023, the Florida Health Department has been reporting travel-related and locally acquired dengue cases. Dengue is a vectorborne infectious disease spread by infected mosquitoes and endemic in about 125 countries.
During week #40, Florida reported 32 new dengue cases.
As of October 7, 2023, Florida's Arbovirus Surveillance report confirmed 17 new travel-associated Dengue cases. In 2023, there have been 351 dengue cases associated with international travelers, led by Miami-Dade County with 207.
Over sixty-two percent (220) of these dengue cases are related to visitors from Cuba.
Of more concern to health officials is the recent increase in locally acquired dengue cases.
Furthermore, there were 15 new cases of locally acquired dengue last week. This data increases 2023's total to 53 cases, with Miami-Dade County reporting 47 locally acquired cases.
According to the Centers for Disease Control and Prevention, there have been 1,289 dengue cases reported by 48 U.S. jurisdictions in 2023. In addition to Florida, New York has reported 90 dengue cases this year.
About 3.4 million dengue cases have been reported in the Region of the Americas so far this year.
As of October 11, 2023, two dengue vaccines are available globally, but only one is licensed in the U.S. Sanofi Pasteur's Dengvaxia® vaccine is available for certain people following a diagnostic test review.

The European Commission (EC), the European Investment Bank (EIB), and the Bill & Melinda Gates Foundation today announced a new financing partnership focused on eradicating polio and ensuring that innovations in health are more accessible to the people.
Confirmed on October 11, 2023, the expected $1.1 billion financing package aims to provide new funding to eradicate a human disease for only the second time in history: polio.
As implementing partners for the polio funding, the World Health Organization (WHO) and UNICEF will deploy the resources to eradicate polio, support the distribution of other childhood immunizations, and strengthen health systems to better respond to emerging health threats.
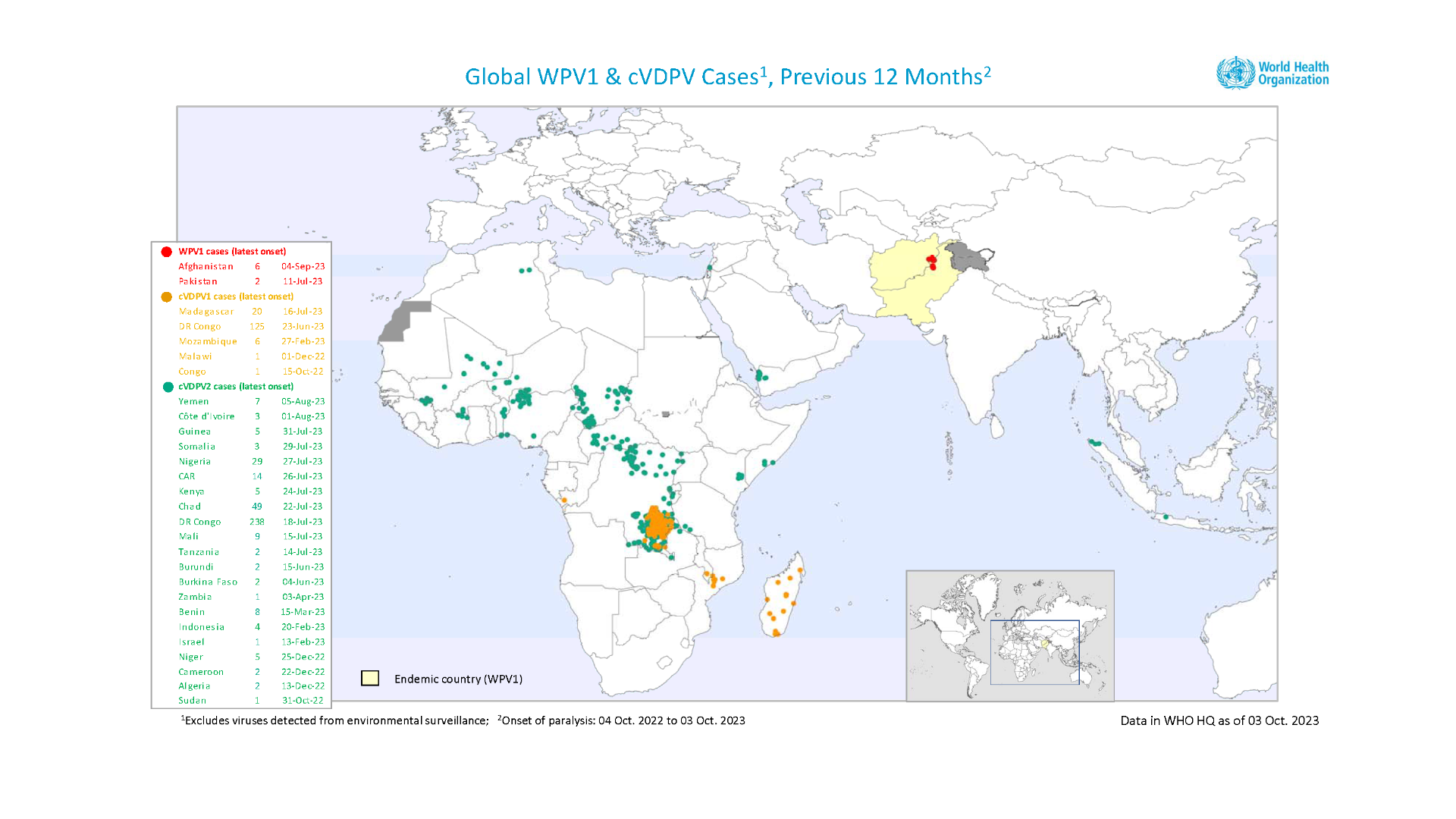
According to the Global Polio Eradication Initiative (GPEI), there are 35 countries with poliovirus detections, two polio-endemic endemic countries, and eight at-risk countries as of August 2023.
The total number of poliomyelitis samples collected in countries with poliovirus transmission was 12,259 from 40 countries in 2022, an increase from 36 countries in 2021.
These poliovirus detections included the southern part of New York.
President of the European Commission Ursula von der Leyen commented in a related press release, "We are about to wipe polio off the face of the Earth."
"The EC, the EIB and the Bill & Melinda Gates Foundation are partnering to get through the final stretch."
"With 1 billion euros supported by our European investment strategy Global Gateway, we will invest in stronger health systems globally and local vaccine and medicines production, manufacturing and administration, where it is most needed."
According to the WHO and the U.S. Centers for Disease Control and Prevention's updated Global Polio Alert - Level 2, Practice Enhanced Precautions Travel Health Notice, issued on September 11, 2023, polio outbreaks and poliovirus detections. are expected to continue.
The WHO confirmed on August 25, 2023, that the spread of poliovirus remained a Public Health Emergency of International Concern and extended the ongoing emergence for an additional three months.
There are several polio vaccines available worldwide in 2023. In the U.S., polio vaccines are generally available at health clinics and pharmacies.
Furthermore, the WHO and GPEI have led the distribution of about 750 million nOPV2 vaccines in Africa over the past few years.
During the 2023 Grand Challenges Annual Meeting, Bill Gates, Co-chair of the Bill & Melinda Gates Foundation, announced new $40 million investments to advance access to mRNA research and vaccine manufacturing technology that will support low- and middle-income countries (LMICs) capacity to develop high-quality, lifesaving vaccines.
Announced on October 9, 2023, this new funding builds on the Gates Foundation's previous $55 million investment in mRNA manufacturing technology.
"Expanding the availability of affordable, high-quality vaccines that meet the needs of local communities is one of the best ways to improve global health outcomes and reduce preventable deaths," commented Trevor Mundel, president of the Gates Foundation's Global Health Division, in a press release.
"By lowering barriers to access for LMICs, we can help ensure more people around the world benefit from lifesaving health innovation."
The foundation's funding advances access to Quantoom Biosciences' low-cost mRNA research and manufacturing platform, developed with an early-research Grand Challenges grant made to its parent company, Univercells.
The Institut Pasteur de Dakar and Biovac, research institutes with vaccine manufacturing experience based in Senegal and South Africa, respectively, will receive US$5 million each to acquire the technology and will be able to use it to develop locally relevant vaccines.
To further advance the technology and lower costs for commercialization, the foundation will also provide US$20 million to Quantoom Biosciences, ensuring LMICs can benefit from the next-generation mRNA health tools.
The Gates Foundation will grant another US$10 million to other LMIC vaccine manufacturers to be named.

New strategies to improve flu shot uptake were recently deployed during the recent pandemic in the United States.
Despite the clearly defined risks to children from influenza, pediatric flu shot rates have been suboptimal.
A new study published in the journal Pediatrics evaluated the impact of an influenza vaccine mandate on schoolchildren's behavior in Massachusetts during the 2020–2021 flu season.
In August of 2020, Massachusetts mandated that all children aged six months and older attending daycare through college virtually or in person receive an influenza vaccine, allowing for certain exemptions.
However, Massachusetts's neighboring states of Maine and New Hampshire did not adopt similar mandates.
Published on October 10, 2023, this study found children, mean age of 9.7 years, had a higher predicted probability of receiving an influenza vaccine than those living in states without a mandate (47.7%, confidence interval 46.4%–49.0%, vs. 21.2%, confidence interval 18.8%–23.6%, respectively, for previous non-vaccinators.
And 78.2%, confidence interval 77.4%–79.0%, vs 58.2%, confidence interval 54.7%–61.7%, for previous vaccinators).
The difference was 6.5% greater among previous nonvaccinators (confidence interval 1.3%–11.7%).
Previously vaccinated children had a lower predicted probability of receiving an influenza vaccine if they lived in a county with the highest COVID-19 severity compared with a county with low COVID-19 severity (72.1%, confidence interval 70.5%–73.7%, vs 77.3%, confidence interval 74.7%–79.9%).
These researchers concluded that an influenza vaccine mandate appears to be an effective but insufficient strategy to achieve optimal vaccine coverage rates.
For the 2023-2024 flu season, over 116 million flu shots have been distributed throughout the U.S. About 173 million influenza vaccines were distributed in the U.S. during the 2022-2023 flu season.
Furthermore, the U.S. CDC recommends an annual flu shot for most people early in the evolving flu season. These vaccines are offered at most health clinics and pharmacies in the U.S.
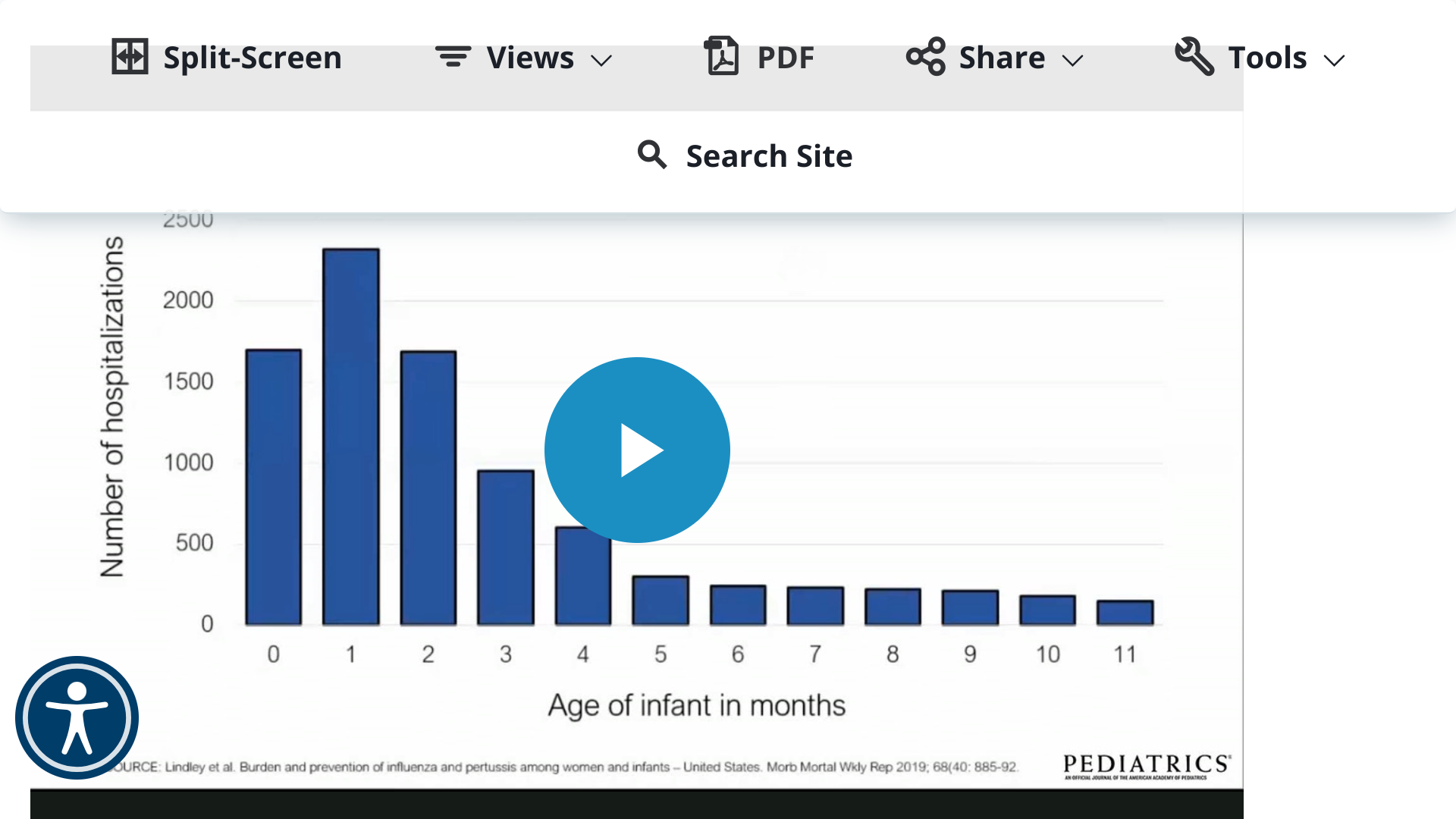
Pertussis vaccination during pregnancy has been implemented in many high-resource countries, and recent data from the United States, the United Kingdom, and South America demonstrate its effectiveness in reducing infant pertussis in the first two months of life.
What has not been well established is whether 'blunting' (maternal immunological interference) has clinical consequences.
Blunting of the infant’s subsequent response to primary immunization by maternally derived antibodies has been demonstrated for many antigens.
A new study published in the journal Pediatrics disclosed pertussis vaccination near 28 weeks' gestation was associated with a lower risk of infection among infants through 8 months of age.
These Australian researchers reviewed records from 2013 through 2017 to calculate the impact after infants received diphtheria, tetanus, and pertussis DTaP doses.
The vaccine effectiveness (VE) from maternal vaccination in infants younger than two months was 70.4% (95% confidence interval 50.5% to 82.3%).
Published on October 9, 2023, these researchers observed slightly lower VE point estimates for the third dose of infant pertussis vaccine among maternally vaccinated compared with unvaccinated infants (76.5% vs. 92.9%, P = .002) and did not observe higher rates of pertussis infection (hazard ratio, 0.70; 95% CI, 0.61–3.39).
The vaccine was given usually between 28 to 31 weeks gestation.
A commentary by Kathryn Edwards, MD, professor of pediatrics at Vanderbilt University Medical Center, stated, 'The consequences of maternally derived antibody on infant responses will need to continue to be monitored, as was done in the carefully conducted study of pertussis reported in this issue of Pediatrics. It will be critical to assess the burden of vaccine-preventable diseases and affirm that blunting from maternal immunization has no material impact on disease control.'
World Polio Day is an opportunity to highlight efforts toward a polio-free world and honor the tireless contributions of those on the frontlines in the fight to eradicate polio from every corner of the globe.
The Global Polio Eradication Initiative (GPEI) confirmed World Polio Day is on October 24, 2023, and has launched a Make Polio History campaign to rally existing and new supporters of polio eradication..... through vaccination programs.
Over the past few years, the GPEI has led the deployment of the type 2 novel oral polio vaccine (nOPV2), which is genetically more stable than existing oral polio vaccines, with a lower risk of reversion to neurovirulence.
Approximately 750 million nOPV2 doses have been administered in more than 35 countries worldwide.
Unfortunately, polio outbreaks have been confirmed in various countries in 2023, led by Afghanistan and Pakistan.
The World Health Organization confirmed on August 25, 2023, that the spread of poliovirus remained a Public Health Emergency of International Concern and extended the emergency notice for an additional three months.
"It's Time to Make Polio History,' says U.S. CDC Director Mandy K. Cohen, MD, MPH, in a related video message posted on YouTube on October 4, 2023. "It won't be easy, but making history never is. We have the tools and knowledge to ensure no child is paralyzed by polio again."
In the United States, the IPV polio vaccine is offered at most health clinics and pharmacies.

LimmaTech Biologics AG announced today the closing of a USD 37 million Series A financing round that will empower its proprietary technology platform and accelerate its preclinical and clinical vaccine candidates against increasingly dangerous bacterial infections, including programs addressing shigellosis and gonorrhea.
Antimicrobial Resistance is responsible for approximately 5 million deaths annually. Infections that were once easily treatable have now become difficult, if not impossible, to cure.
As a leading example of this threat to global health, half of the approximately 700,000 annual gonorrhea infections in the U.S. are already resistant to antibiotics, and there is a real threat of gonorrhea soon becoming untreatable.
While there are no gonorrhea vaccines available, off-label vaccines and treatments are in use.
Later-stage clinical development efforts will focus on the company's Shigella vaccine program, which LimmaTech developed with GSK. The company expects to announce preliminary results from the Shigella program's ongoing Phase 2 clinical trial in the second half of 2023.
Shigella cause an estimated 450,000 infections in the U.S. each year.
According to the U.S. CDC, people can get a Shigella infection (shigellosis) after putting something in their mouth or swallowing something that has come into contact with the stool of someone with a Shigella infection.
"Within the next decade, multiple bacterial infections will become untreatable due to antimicrobial Resistance, which is already a significant burden on global health. By advancing our innovative technology platform, LimmaTech has the potential to simultaneously provide vaccine-induced protection against bacterial infections, mitigate the increasing risk of antibiotic resistance, and move toward the control of several highly transmissible pathogens," commented Dr. Franz-Werner Haas, CEO of LimmaTech, in a press release on October 9, 2023.
".....With this support and our team of proven experts in bacterial vaccine development and manufacturing, we look forward to providing life-changing vaccines to address a major global medical need."
The Company is conducting a Phase I/II clinical trial in the Republic of Kenya of a 4-valent candidate vaccine to help prevent diarrheal disease caused by the Shigella bacteria in children and infants in low and middle-income regions. The Shigella study is conducted in collaboration with GSK and the Wellcome Trust.
LimmaTech is committed to translating novel scientific concepts into highly effective vaccines that benefit humanity. For more information, please visit www.lmtbio.com.


