Search API
A seldom-discussed diarrheal disease that affects millions of children and adults may soon have a preventive vaccine available. Furthermore, the World Health Organization has identified Shigella prevention as a priority.
LimmaTech Biologics AG today announced that it entered into a strategic partnership and exclusive licensing agreement with Valneva SE for the development, manufacturing, and commercialization of Shigella4V (S4V), a tetravalent bioconjugate vaccine candidate against Shigellosis.
As of August 1, 2024, no approved Shigella vaccine is available.
However, the global market opportunity for a vaccine against Shigella is estimated to exceed $500 million annually.
Shigellosis, caused by Shigella bacteria, is the second leading cause of fatal diarrheal disease worldwide. It is estimated that up to 165 million cases of disease and an estimated 600,000 deaths are attributed to Shigella each year.
Moreover, Shigellosis affects international travelers and military personnel in endemic regions.
Under the terms of the agreement with Valneva, LimmaTech confirmed it will receive an upfront payment of €10 million ($10.8m) and be eligible to receive additional regulatory, development, and sales-based milestone payments as well as low double-digit royalties on sales.
LimmaTech will be responsible for conducting a Phase 2 Controlled Human Infection Model and a Phase 2 pediatric study. Both clinical trials are expected to begin in the second half of 2024.
Dr. Franz-Werner Haas, LimmaTech's Chief Executive Officer, said in a press release, “Having developed the S4V Shigella vaccine candidate from its early discovery phase to the promising clinical data we have achieved to date, we are excited to accelerate the program with our partnership with Valneva."
"This agreement underscores our capabilities to leverage LimmaTech’s proficiency in vaccine development with the best path to develop programs rapidly."
LimmaTech initiated the tetravalent Shigella vaccine candidate and continued to lead its development as part of its ongoing collaboration with GSK. Later, it in-licensed the vaccine candidate from GSK.
In February 2024, LimmaTech reported positive interim Phase 1/2 data for the S4V vaccine candidate, including a favorable safety and tolerability profile and robust data on immunogenicity against the four most common pathogenic Shigella serotypes: S. flexneri 2a, 3a, 6, and S. sonnei4.
LimmaTech Biologics AG LimmaTech Biologics is at the forefront of combating the global antimicrobial resistance epidemic based on its unparalleled track record in vaccine technology and clinical candidate development. The company is leveraging its proprietary self-adjuvanting and multi-antigen vaccine platform alongside additional disease-specific vaccine approaches to prevent increasingly untreatable microbial infections.

Merck today announced financial results for the second quarter of 2024 were $16.1 Billion, an increase of 7% from the same period in 2023.
In the cancer prevention market segment, Merck's Human papillomavirus (HPV) vaccines reported sales increases.
The 4% growth in the GARDSAIL/GARDASIl 9 HPV vaccines was primarily due to higher sales in the U.S., driven by higher pricing, demand, public-sector buying patterns, and higher demand in certain ex-U.S. markets.
The growth was largely offset by lower sales in China due to the timing of shipments compared with the prior year.
“Our business is demonstrating strong momentum as we exit the first half of the year,” said Robert M. Davis, chairman and chief executive officer of Merck, in a press release on July 30, 2024.
“Through excellent scientific, commercial, and operational execution, we’re achieving significant milestones for our company and patients, including the launch of WINREVAIR. I am proud of our dedicated teams around the world that are working tirelessly to advance our deep pipeline as we continue delivering innovation that solves unmet medical needs.”
According to the U.S. CDC, there is an increasing rate of STDs. For example, people 55 and older reported a significant increase in HPV diagnoses.
'Almost every unvaccinated person who is sexually active will get HPV at some time in their life. About 13 million Americans, including teens, become infected with HPV each year. Most HPV infections will go away on their own. But infections that don’t go away can cause certain types of cancer.'
Merck's HPV vaccines are generally available at medical clinics and pharmacies in the United States.

Versatope Therapeutics Incorporated announced today it has received a Phase 2 Small Business Innovation Research (SBIR) grant for up to $3 million over three years from the U.S. NIH's National Institute of Allergy and Infectious Diseases.
On July 29, 2024, Versatope confirmed it will use the grant (#R44AI181242) to develop a bi-specific malaria vaccine using a target that blocks the initial malaria infection and transmission.
The Company says the novel, dual-acting vaccine may offer a more robust approach than the current World Health Organization (WHO) certified single-acting malaria vaccines.
Versatope was also awarded a Stage I grant from the MassVentures SBIR Targeted Technologies program.
"We appreciate the recognition and support of the NIH and MassVentures team to advance the development of Versatope's technology platform and to help take the company to the next stage of development," said Christopher Locher, CEO of Versatope, in a press release.
As of July 2024, two malaria vaccines are being deployed in various countries.
For example, the African country of Côte d'Ivoire recently became the first nation to deploy the R21/Matrix-M™ vaccine.
"The introduction of the R21/Matrix-M™ malaria vaccine in Côte d'Ivoire marks a breakthrough in the fight to protect vulnerable children against a leading cause of death across the region while reinforcing our mission to create innovative vaccines that improve public health," said John Jacobs, Novavax Inc.'s President and CEO, said in a press release on July 15, 2024.
A new World Health Organization (WHO) project aims to accelerate the development and accessibility of human avian influenza (H5N1) messenger RNA (mRNA) vaccine candidates.
The Argentinian manufacturer Sinergium Biotech is leading this effort, leveraging the WHO and the Medicines Patent Pool mRNA Technology Transfer Programme.
Sinergium Biotech has developed candidate H5N1 vaccines and aims to establish proof-of-concept in preclinical models. Once the preclinical data package is concluded, the technology, materials, and expertise will be shared with other vaccine manufacturing partners, accelerating the development of H5N1 vaccine candidates and bolstering pandemic preparedness efforts.
The mRNA Technology Transfer Programme, jointly developed by WHO and MPP, was launched in July 2021.
"This initiative exemplifies why WHO established the mRNA Technology Transfer Programme – to foster greater research, development, and production in low- and middle-income countries so that when the next pandemic arrives, the world will be better prepared to mount a more effective and more equitable response," said Dr. Tedros Adhanom Ghebreyesus, WHO Director-General, in a press release on July 29, 2024.
Avian influenza viruses are a public health risk due to their global circulation in birds and mammals. According to the WHO, they have the potential to cause a future human pandemic.
However, the WHO says avian influenza viruses are currently a low risk for most people.
In the United States, avian influenza vaccines have been developed, candidates are under development, and one has been U.S. FDA-approved.

About 10% of children in Gavi-supported countries do not receive a single dose of routine vaccines.
To reach these missing millions, Gavi announced on July 11, 2024, that the 5.0 Strategy intends to reduce the number of zero-dose children by 25% by 2025 and 50% by 2030.
The new strategy will focus on reaching the most marginalized by strengthening primary healthcare systems, building and sustaining community demand, addressing gender barriers, and using innovation to ensure immunization services reach these children.
Available data suggests the largest disruptions were concentrated in Q2 2020, with the majority of countries restoring routine immunization services in the second half of the year. Over 75% of under-immunised children are now zero-dose, heightening the risk of child deaths, disease outbreaks, and medical impoverishment.
In 2000, just 47% of children in lower-income countries received essential vaccines.
In Gavi-eligible countries, coverage of critical vaccines increased by three percentage points from 2015 to 2019, and the number of zero-dose children was reduced by 14%.
In 2019, coverage for the same countries reached 82% before sliding back to 78% due to the pandemic.
According to data published by the World Health Organization and UNICEF, in 2022, 20.5 million children in India missed out on one or more vaccines delivered through routine immunization services.
Gavi wrote, 'The importance of immunization reaching all children is paramount and ensures all children have an equal chance of being healthy and productive members of society.'
'Vaccination, because of its preventative nature, averts illness and provides particularly significant benefits to zero-dose communities which may lack access to affordable, quality curative care while being at higher risk of vaccine-preventable diseases.'
'Immunised children are also more likely to grow up healthy and enjoy their survival and development rights. Vaccinated children have higher cognitive abilities, miss school less and are in school for longer, and have better nutrition and education outcomes – all of which translates into better-earning potential and productivity as an adult.'

Globally, respiratory syncytial virus (RSV) is the leading cause of hospitalization for healthy infants under a year old and causes an estimated 101,000 deaths a year in children under five.
To address this significant health risk, Merck today announced positive topline results from its Phase 2b/3 clinical trial evaluating clesrovimab (MK-1654), the company’s investigational prophylactic monoclonal antibody (mAb) designed to protect infants from respiratory syncytial virus (RSV) disease.
Clesrovimab met its primary safety and efficacy endpoints in the trial, including reducing medically attended lower respiratory infections caused by RSV through Day 150.
Clesrovimab is being studied in infants (pre-term and full-term) to provide rapid, durable protection through their first RSV season with a single, fixed-dose administration.
“RSV is highly contagious and can cause inflammation in the airways of infants, leading to difficulty breathing. As a widespread illness globally, RSV is the leading cause of hospitalization for healthy infants,” said Dr. Paula Annunziato, senior vice president of infectious diseases and vaccines, Global Clinical Development, Merck Research Laboratories, in a press release on July 23, 2024.
“We are encouraged by these findings and look forward to working with regulators to provide a new option to help address the impact of RSV on infants and their families."
For the 2024-2025 RSV season, the U.S. FDA-approved Beyfortus™ (Nirsevimab) mAb offers passive immunization to prevent lower respiratory tract infections caused by the RSV to infants experiencing their first or second RSV season and those with congenital heart disease or chronic lung disease.
Additionally, one vaccine has been approved for pregnant women, which offers RSV protection to newborns.

With over 24,600 Zika virus cases already confirmed in the Region of the Americas in 2024, a new study published by the journal eBioMedicine, part of The Lancet, disclosed new, unsettling insights.
The Cleveland Clinic announced that this study revealed that maternal Zika virus infections can reprogram fetal immune development, leading to long-term consequences in children's immunity.
On July 18, 2024, the Clinic stated that these changes may occur in children born without the physical characteristics associated with congenital Zika syndrome, such as microcephaly.
This finding suggests that 95% of babies born from Zika-infected pregnancies who did not exhibit symptoms may have been affected by the virus with long-term immunological repercussions.
Additionally, heightened inflammation was observed in Zika-exposed infants with abnormalities at birth, while children exposed to Zika later maintained a chronic Th1-biased immune profile. The impaired response to Th2-biased vaccines raises concerns about the lasting effects of Zika virus exposure on immune responses.
Suan-Sin (Jolin) Foo, PhD, an expert in maternal-fetal virology and the Zika virus, says babies without symptoms are deemed healthy and do not receive any follow-up medical care or attention.
“Studies have only really focused on what’s happening with the children who were born with visible physical conditions like microcephaly or neurological complications,” Dr. Foo commented in a press release.
“The rest of these kids may not even have a note on their chart mentioning that their mother was infected during pregnancy. Unless they’re part of our study, they’re essentially lost to the medical field.”
Dr. Foo added, “Our study clearly shows there’s much more to this condition than meets the eye. We need to expand diagnostic criteria and conduct more research to ensure these immunologically vulnerable children get the necessary care.”
From a prevention perspective, no Zika vaccine candidate has been approved by the U.S. FDA as of July 2024.

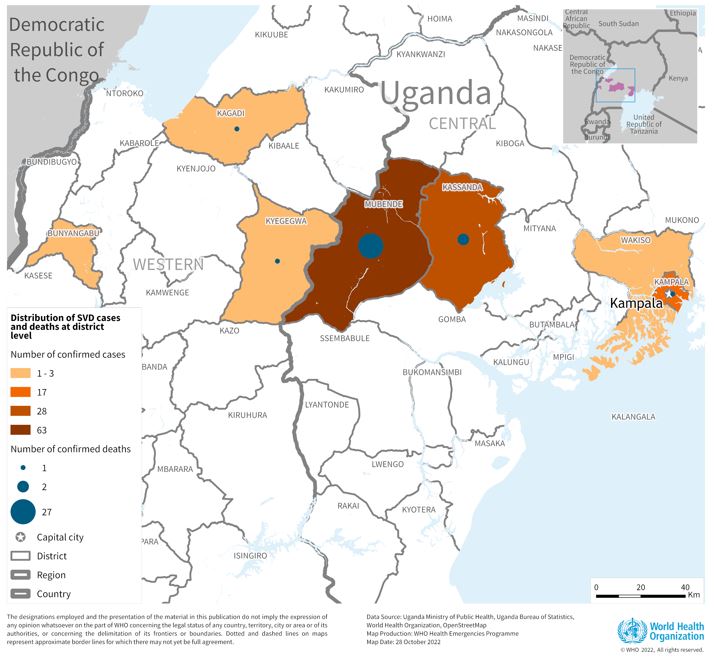
The Sabin Vaccine Institute recently announced the launch of a Phase 2 clinical trial for its vaccine against Sudan ebolavirus at Makerere University Walter Reed Project (MUWRP) in Uganda.
This is a vital development as there are currently no approved vaccines for this strain of ebolavirus.
Based on the cAd3 platform, Sabin’s single-dose investigational Sudan ebolavirus vaccine was found to be promising in Phase 1 clinical and non-clinical studies. Results showed it to be safe while eliciting rapid and robust immune responses that lasted up to 12 months.
“We are delighted to advance a vaccine candidate that can thwart a deadly and devastating disease, especially one that caused a fairly recent outbreak and for which no approved treatments exist,” commented Amy Finan, Sabin’s Chief Executive Officer, in a press release on July 15, 2024.
“Sabin’s vaccine candidate is backed by strong safety and immunogenicity data, and we hope this trial will yield further evidence to move the vaccine closer to licensure.”
This is Sabin’s second Phase 2 clinical trial partnership with MUWRP, based in Uganda’s capital, Kampala. A Phase 2 trial for a Marburg vaccine is already underway, having recently completed enrollment. Initial results from the Marburg trial are expected later this year.
The most recent outbreak of Sudan ebolavirus occurred in Uganda in the fall of 2022. That outbreak ultimately resulted in 55 deaths.
Sabin’s vaccine candidate was the first to arrive in Uganda during that outbreak after the WHO included it as one of three vaccines for possible use in an outbreak trial. The outbreak ended before the vaccine was deployed.
In August 2019, Sabin announced agreements with GSK to advance the development of vaccines against the Zaire and Sudan ebolavirus and Marburg virus. The three candidate vaccines were initially developed collaboratively by the U.S. National Institutes of Health and Okairos, acquired by GSK in 2013.
As of July 20, 2024, the U.S. FDA has approved Zaire Ebolavirus vaccines, which have been offered in Africa since 2019.