Search API
Tuberculosis (TB) is not only the world's biggest infectious killer, but it also destroys families and livelihoods. Besides the fear of dying from the disease, in many communities, a diagnosis can sentence someone to social isolation, wrote Linda Geddes in an article published by Gavi, the Vaccine Alliance, on October 24, 2023.
While the Bacillus Calmette–Guérin (BCG) vaccine provides significant protection against TB disease in infants and young children, new vaccines that block infection and prevent TB disease are urgently needed.
More than 100 years have passed since the first administration of the BCG vaccine; hopes are building that a vaccine that could protect all age groups against all types of TB may finally be in reach.
Fortunately, several TB vaccine candidates are now in late-stage clinical trials, raising hopes that an affordable and effective vaccine may soon be within reach.
Today's BCG vaccines are based on different attenuated strains of M. bovis.
These BCG vaccines are recommended for newborns in countries with a high burden of TB.
TB vaccines are among the most widely used, reaching more than 80% of infants in countries where the BCG vaccine is included in routine childhood immunisation programs.
In the United States, vaccinations with the TICE® BCG version are limited and generally offered to children in areas experiencing community spreading of TB.
The U.S. CDC reported in March 2023 that TB cases increased by 5% in 2022, with 8,300 confirmed cases. In addition, about 13 million people live with latent TB infection in the U.S.
Recent data indicates TB rates are accelerating by double digits in certain Texas areas (Dallas, Hidalgo County, Houston, San Antonio) in 2023.
Gedde's full, unedited article is posted at this link.

While the World Health Organization (WHO) Influenza Update N° 456 says respiratory syncytial virus (RSV) activity was generally low or decreasing globally, new data indicates the United States is seeing measurable increases.
The U.S. Centers for Disease Control and Prevention (CDC) RSV detection graphs showed increases throughout the U.S. as of October 27, 2023.
The National Respiratory and Enteric Virus Surveillance System (NREVSS) reports that the weekly percentage of RSV polymerase chain reaction (PCR) test positivity was 10.9% as of October 21, 2023.
During the 2022–2023 RSV season, positive test results peaked in November.
From an age perspective, the Weekly Emergency Department Visits chart clearly indicates that the most affected by this RSV season have been children under one-year-old.
In 2022, the JAMA Network conducted an Original Investigation that found 96 (95% CI, 92-99) RSV deaths among children younger than one year. And a study published by the Journal of Infectious Diseases determined that RSV-related deaths in infants <1 year peaked at about one month.
Furthermore, a meta-analysis of eleven studies published in October 2023 found that the meta-estimate of RSV-positive tests among pregnant women was 3.4% (95% CI: 1.9; 54).
According to the CDC, RSV has geographic trends in the U.S.
Florida's RSV season is longer than the rest of the country and has distinct regional patterns.
The Florida Department of Health reported as of week #42, October 21, 2023, RSV activity was increasing in hospital admissions and emergency room rates, with current outbreaks in Martin (1), Pinellas (2), and Volusia (1).
Fortunately, for the first time, there are approved RSV vaccines and passive immunizations available in the U.S. this season.

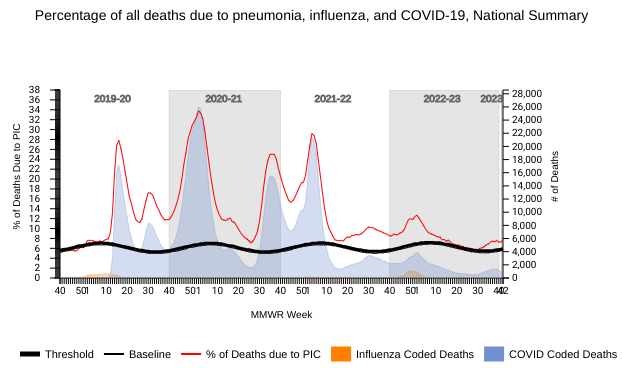
The U.S. National Center for Health Statistics (NCHS) Mortality Surveillance reported on October 27, 2023, that most respiratory disease deaths last week were related to pneumonia, not COVID-19 or influenza.
During week #42, the NCHS reported 1,379 pneumonia deaths, compared with 637 COVID-19 and 20 influenza.
While these diseases have U.S. FDA-approved vaccines available in 2023, preventing pneumonia deaths is more complicated, as viruses, bacteria, and fungi can all cause pneumonia.
According to the U.S. CDC, pneumonia is an infection of the lungs that can cause mild to severe illness in people of all ages.
Globally, pneumonia claimed the lives of 2.5 million people, including 672,000 children, in 2019 alone.
Vaccines can help prevent most pneumonia cases caused by pneumococcus bacteria, SARS-CoV-2, or influenza viruses.
These immunizations include
- COVID-19
- Haemophilus influenzae type b
- Influenza
- Measles
- Pertussis
- Pneumococcal
- Respiratory syncytial virus
- Varicella
According to the CDC, these immunizations are safe and may be coadministered. But side effects can occur which are usually mild and go away on their own within a few days.
These vaccines are generally available at health clinics and pharmacies in the U.S.
Note: The NCHS data presented on October 27, 2023, are preliminary and may change as more data are received and processed.
Moderna, Inc. recently announced that the first participant has been dosed in a Phase 3 study of the Company's combination vaccine candidate against influenza and COVID-19 (mRNA-1083) in the U.S.
As of October 24, 2023, the Phase 3 study is expected to enroll approximately 8,000 adults and will evaluate the immunogenicity, safety, and reactogenicity of mRNA‐1083 as compared with active control, co‐administered licensed influenza and SAR‐CoV‐2 vaccines in two independent age‐group sub‐study cohorts.
The mRNA-1083 candidate selected to advance to Phase 3 achieved hemagglutination inhibition antibody titers similar to or greater than those of both licensed quadrivalent influenza vaccines and SARS-CoV-2 neutralizing antibody titers similar to those of the Spikevax bivalent booster in the Phase 1/2 study.
mRNA‐1083 has the potential to efficiently reduce the overall burden of acute viral respiratory diseases by providing simultaneous protection against influenza and SARS‐CoV‐2 viruses in a single injection.
mRNA‐1083 offers greater convenience and has the potential to lead to increased compliance with vaccine recommendations.
This approach could benefit public health by synergistically increasing coverage rates against influenza and SARS‐CoV‐2 viruses.
The Company continues to target a potential initial regulatory approval for the combination vaccine in 2025.

Araclon Biotech recently announced encouraging final results from its Phase 2 clinical trial of ABvac40, an active vaccine against the Aβ40 peptide, for treating patients with early-stage Alzheimer's disease (AD).
The results show that ABvac40 had a favorable safety profile, elicited a robust immune response against Aβ40, and demonstrated some potential cognitive benefits in early-stage AD patients.
The vaccine candidate met the primary endpoints and showed differences between the vaccine- and placebo-treated groups in some secondary exploratory endpoints.
ABvac40 is uniquely designed to target the C-terminal end of the Aβ40 peptide.
Thus, it is believed to prevent harmful reactions and avoid immune triggers responsible for meningoencephalitis, a complication observed in earlier AD vaccines.
Emerging research suggests that Αβ40 plays a role in cerebral amyloid angiopathy, a highly prevalent condition among the growing number of AD patients.
Notably, although the clinical trial was not powered for finding efficacy on neuropsychological scales, the ABvac40-treated group exhibited as much as a 38% reduction in disease progression, as reflected by the Mini-Mental State Examination score, suggesting ABvac40's potential efficacy in addressing the cognitive decline associated with AD.
Other neuropsychological tests, such as the Repeatable Battery for the Assessment of Neuropsychological Status and the Trial Making Test, showed favorable results on ABvac40 compared to the placebo group.
Global or functional scales did not show differences between the ABvac40 and placebo groups. In addition, volumetric magnetic resonance imaging showed a lesser increase in whole-brain atrophy in the ABvac40 group compared to the placebo group.
"We are pleased to report final positive results from the Phase 2 study of ABvac40, including a robust immune response with some significant reduction in disease progression, all with a favorable safety profile," said Jose Terencio, Ph.D., Araclon chief executive officer and vice president of Grifols Innovation and New Technologies, in a press release on October 25, 2023.
"Previous vaccines in development for AD faced setbacks due to harmful meningoencephalitis side effects."
"The results reported for ABvac40 to date validate its clinical potential, positioning it as a promising therapeutic candidate for early AD treatment. We look forward to evaluating the next steps for this program."
As of October 27, 2023, the U.S. FDA has not approved a vaccine targeting AD.

Pfizer Inc. and BioNTech SE today announced positive topline results from a Phase 1/2 clinical study evaluating the safety, tolerability, and immunogenicity of mRNA-based combination vaccine candidates for influenza and COVID-19 among healthy adults.
The data from the trial showed that the companies' lead formulations demonstrated robust immune responses to influenza A, influenza B, and SARS-CoV-2 strains.
The topline results of the ongoing trial demonstrated that the combination formulations evaluated had a safety profile consistent with the safety profile of the companies' COVID-19 vaccine.
In the Phase 1/2 clinical trial, the vaccine candidates were compared to a licensed influenza vaccine and the companies' Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine given at the same visit.
A pivotal Phase 3 trial evaluating these lead formulations is expected to be initiated in the coming months.
"Studies of confirmed viral infections suggest that COVID-19 adopts a seasonal pattern with peaks in fall and winter, similar to other respiratory diseases. Co-infections and consecutive respiratory infection during this period can further increase the risk of severe illness," said Prof. Ugur Sahin, MD, CEO and Co-founder of BioNTech, in a press release on October 26, 2023.
"Combination vaccines have the potential to become a mainstay of routine vaccination against respiratory diseases, especially for the vaccination of populations with a higher risk of severe illness."
Immunogenicity results induced by lead formulations in the companies' phase 1/2 trial showed point estimates for Geometric Mean Titer (GMT) ratios consistent with the criteria applied to regulatory-approved vaccines against the respective influenza and SARS-CoV-2 strains.
Point estimates for GMT ratios for all matched influenza vaccine strains with lead formulations were >1 relative to a licensed Quadrivalent Influenza Vaccine given concomitantly with the Pfizer-BioNTech COVID-19 vaccine.
Pfizer and BioNTech previously announced that their mRNA-based combination vaccine candidate for influenza and COVID-19 received Fast Track Designation from the U.S. Food and Drug Administration.
For the 2023-2024 flu season in the U.S., 136.94 million influenza vaccine doses had been distributed as of October 14, 2023.

Bavarian Nordic A/S today announced the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) voted in favor of recommending the routine use of JYNNEOS® (MVA-BN®), the Company’s U.S. FDA-approved mpox / smallpox vaccine.
Specifically, the ACIP voted to recommend that individuals 18 years and older with certain risk factors receive the two-dose JYNNEOS regimen, a live, nonreplicating vaccine.
Previously, the ACIP had recommended JYNNEOS for individuals at risk during a mpox outbreak.
This represents the second national recommendation for Bavarian Nordic’s mpox vaccine in adult risk groups following a similar endorsement by the Standing Committee for Vaccination in Germany in 2022.
More recently, the European AIDS Clinical Society also recommended using the vaccine for adults infected with HIV or on pre-exposure prophylaxis treatment, which may support additional national recommendations for future vaccine use.
“Since the outbreak of mpox last year (May 2022), Bavarian Nordic has supplied millions of doses of our vaccine to more than 70 countries worldwide,” said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release on October 25, 2023.
The CDC estimates that 2 million U.S. individuals are eligible for a vaccination against mpox under this recommendation.
To date, approximately 23% of this group has received the recommended two doses of JYNNEOS, leaving a significant number of people vulnerable to infection with mpox.
The ACIP reported in October 2023 JYNNEOS Vaccine Effectiveness against mpox ranges from 36% to 75% for 1-dose vaccination and 66% to 89% for 2-dose vaccination.
Pending approval of the updated recommendations, Bavarian Nordic is targeting a commercial launch of JYNNEOS in the U.S. in the first half of 2024.

In a study published in The New England Journal of Medicine, clinicians and researchers show that passive immunization by administering plasma taken from convalescent donors after infection with the SARS-CoV-2 coronavirus to patients suffering from acute respiratory distress syndrome requiring artificial mechanical ventilation significantly reduced mortality by 10%.
The effect on reducing mortality was more specifically observed in patients who received convalescent plasma during the first 48 hours after being put on artificial respiratory assistance.
The randomized phase 2 clinical trial involved 17 intensive care units in Belgian hospitals. It included a total of 475 patients from October 2020 to March 2022.
Thanks to the collaboration of the Belgian Red Cross and the laboratories of the KULeuven, UAntwerpen, and ULiège, the intensive care units of the study's partner hospitals were able to use convalescent plasma with high neutralizing antibody titres of 1/320 for 82.3% of patients and 1/160 for the remaining 17.7%.
"For the first time, we have demonstrated the therapeutic value of convalescent plasma in improving these patients' very poor vital prognosis. The reduction in mortality, of the order of 10%, is particularly noticeable in patients who were given convalescent plasma rapidly after the start of artificial respiratory ventilation", explains Dr. Benoît Misset, head of the intensive care unit at the CHU of Liège and Assistant Professor at the Faculty of Medicine at the University of Liège, who is responsible for and first author of this study.
"This study documents and confirms the value of convalescent plasma for passive immunization, but also against possible pathogenic variants and possibly in the event of future pandemics," wrote these researchers on October 25, 2023.