Search API
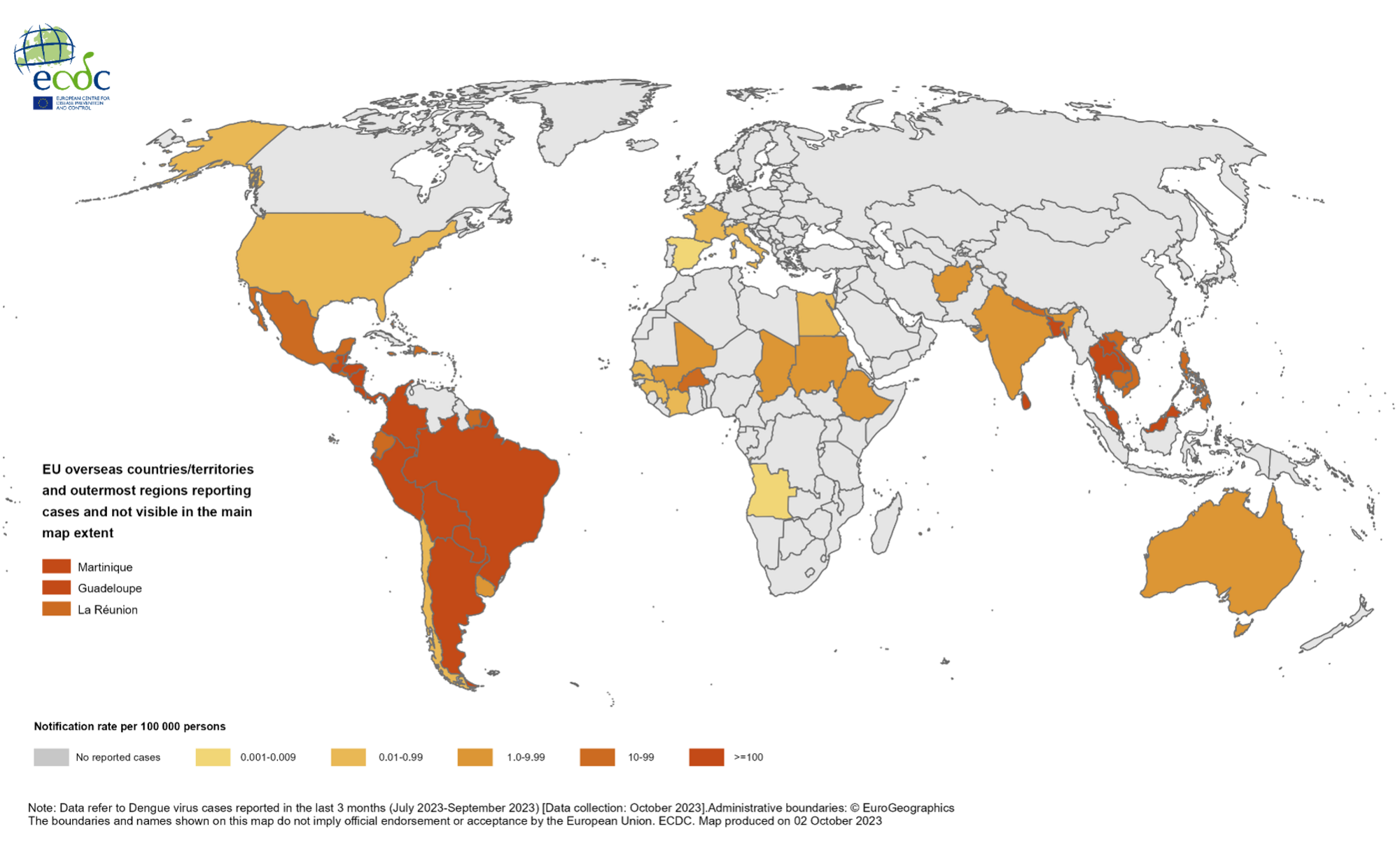
Throughout 2023, the global dengue outbreak has exceeded 4.2 million cases from 79 countries/territories, with the region of the Americas reporting the majority of cases.
Through September 2023, most dengue cases in the region were reported from Brazil and Peru; the latter is experiencing one of the most significant dengue outbreaks in its history, says the European Centre for Disease Prevention and Control.
In the United States, the state of Florida leads in reporting travel-related and locally acquired dengue cases.
As of October 28, 2023, Florida's Health Department confirmed its 513th dengue case of 2023.
Florida's Arbovirus Surveillance Report #43 confirmed 31 new travel-associated dengue cases. In 2023, 415 dengue cases associated with international travelers were reported. Two hundred and six of these cases are related to travel from Cuba.
From a long-term health perspective, health officials are very focused on the increase in locally acquired dengue cases.
Last week, 20 new cases of locally acquired dengue were reported in South Florida, increasing this year's total to 98.
Miami-Dade County has reported 91 of these locally acquired cases.
According to the U.S. Centers for Disease Control and Prevention, dengue is a vaccine-preventable disease that can progress to a severe condition.
As of October 31, 2023, two dengue vaccines are available globally, but only one is licensed in the U.S.
Dengvaxia is U.S. Food and Drug Administration-approved and indicated for preventing dengue virus serotypes 1, 2, 3, and 4. The Dengvaxia® vaccine is available for certain people following a pre-delivery diagnostic test review.
YS Biopharma Co., Ltd. today announced the completion of enrollment in its Phase 3 clinical trial of the PIKA rabies vaccine. The clinical trial will include 4,500 subjects and assess the vaccine's safety, immunogenicity, and lot-to-lot consistency.
Previous Phase 1 and Phase 2 clinical trials of the PIKA Rabies Vaccine have demonstrated its safety and strong immunogenicity, with the PIKA Rabies Vaccine eliciting a detectable immune response in as quick as seven days.
Given these results, the PIKA Rabies Vaccine has the potential to achieve best-in-class accelerated protection and meet the World Health Organization (WHO) goal of a one-week rabies vaccine regimen to replace the conventional three- or four-week regimens.
The PIKA Rabies Vaccine utilizes YS Biopharma's proprietary PIKA adjuvant technology and is designed to produce a more robust immune response in a shorter time span than existing rabies vaccines.
As of October 31, 2023, there are several approved rabies vaccines available.
In a press release, Dr. Zenaida Mojares, Chief Medical Officer of YS Biopharma, commented, "We remain dedicated to leveraging our advanced PIKA adjuvant technology to enhance global health and well-being, and are thrilled to explore the near-term and long-term possibilities it offers."
According to the WHO, rabies has an almost 100% fatality rate upon emergence of clinical symptoms. Each year, it claims the lives of approximately 59,000 individuals in more than 150 countries.
Although rabies is typically lethal without treatment, the administration of post-exposure prophylaxis can effectively prevent fatalities when initiated following possible exposure.
According to the U.S. CDC, human rabies cases are rare in the United States, with only one to three reported annually.
Rabies virus is transmitted through direct contact with saliva or brain/nervous system tissue from an infected animal.
In the U.S., people usually get rabies from the bite of a rabid bat, not dogs.
Rabies vaccination is just one of several travel vaccines recommended when visiting at-risk countries, such as Haiti.

According to Reuters, the new respiratory syncytial virus (RSV) vaccine's usage has surged since its launch in the summer of 2023.
According to IQVIA data seen by Reuters, as of October 30, 2023, GlaxoSmithKline Biologicals (GSK) AREXVY™ RSV vaccine accounts for about two-thirds of RSV immunizations delivered to adults aged 60 years in the United States.
GSK's market advantage could be related to its relationship with CVS Health, the dominant pharmacy chain in the U.S.
In Canada, which approved AREXVY on August 4, 2023, RSV vaccine awareness is leveraging a hockey superstar's endorsement.
"The Arexvy ad featuring Wayne Gretzky aims to dispel the misperception that RSV is only a concern for young children and encourages adults aged 60 and older, who are among those at greater risk, to speak to their healthcare provider about RSV prevention, including the Arexvy vaccine," a GSK spokesperson told Fierce Pharma Marketing yesterday.
In Europe, the European Commission authorized AREXVY in June 2023, followed by the U.K.'s Medicines and Healthcare products Regulatory Agency in July 2023.
On July 26, 2023, GSK estimated that nearly 80 million adults in the U.S. could receive an RSV shot for the first time.
On October 31, 2023, Pfizer Inc. reported U.S. revenues from its RSV vaccine ABRYSVO™, which contributed $375 million since May 2023.
RSV vaccine uptake is influenced by the impact on adults during each RSV season.
The World Health Organization Influenza Update N° 456, published in October 2023, reported early signs of RSV activity in parts of the European region.

Blue Water Biotech, Inc. today issued the following shareholder letter from its CEO, Dr. Neil Campbell.
As of October 30, 2023, Blue Water's vaccine development initiatives highlighted separate indications, such as chlamydia, influenza, malaria, and mpox.
The CEO's letter is excerpted below:
As we close out October, the first month of my tenure, I want to take this opportunity to personally communicate with you while providing an update on the direction of Blue Water Biotech.
As part of the shift in business strategy and to enhance shareholder value, the Company will focus on building a foundation of therapeutic, diagnostic, and service products in oncology that will bolster and enrich clinicians' medical practices.
.... the Company has established early-stage preclinical and clinical programs in various vaccine technologies. These vaccine programs were targeting a wide number of diseases and conditions that would have consumed an enormous amount of Company resources.
Considering the evolving market dynamics and post-pandemic challenges, we conducted a strategic and tactical assessment, which led us to conclude that the optimal path for the Company lies in de-prioritizing the vaccine programs to focus our efforts on expanding our oncology offerings.
In summary, our business strategy shift aims to enhance shareholder value by establishing a strong foothold in both therapeutic and diagnostic aspects of oncology.
The Company's SEC Report of unscheduled material events or corporate events on October 30, 2023, is linked here.

The Journal of Antimicrobial Chemotherapy recently published a study that suggests that influenza vaccinations are associated with less unnecessary antibiotic use in people over 65.
On October 28, 2023, these researchers concluded that seasonal flu shots might be a countermeasure against antimicrobial resistance (AMR) because they can reduce unnecessary antimicrobial use for acute respiratory infection by mitigating the burden of such diseases.
In total, 244,642 people were enrolled in this study in Tokyo, Japan.
The average treatment effect of vaccination was:
−0.004 (95% CI −0.006 to −0.002) for the frequency of antibiotic prescription,
−0.005 (−0.007 to −0.004) for the frequency of healthcare facility consultation,
−0.001 (−0.002 to −0.001) for the risk of admission and
- 0.00 (0.00 to 0.00) for the risk of death.
Our results suggest that the seasonal influenza vaccine might have indirect benefits for not only preventing influenza-like illnesses but also as a countermeasure against AMR, wrote these researchers.
However, because we included only an older population, we cannot know whether a similar effect would be seen if children or young adults were the target population for the vaccine.
Globally, AMR is one of the leading causes of death.
The World Health Organization says that AMR happens when microorganisms, such as bacteria, fungi, viruses, and parasites, change when exposed to antimicrobial drugs, such as antibiotics, antifungals, antivirals, antimalarials, and anthelmintics.
On October 19, 2023, the WHO released 13 interventions to guide country prioritization when developing, implementing, and monitoring national action plans on AMR.
These interventions address the needs and barriers people and patients face when accessing health services through a people-centered approach to AMR.

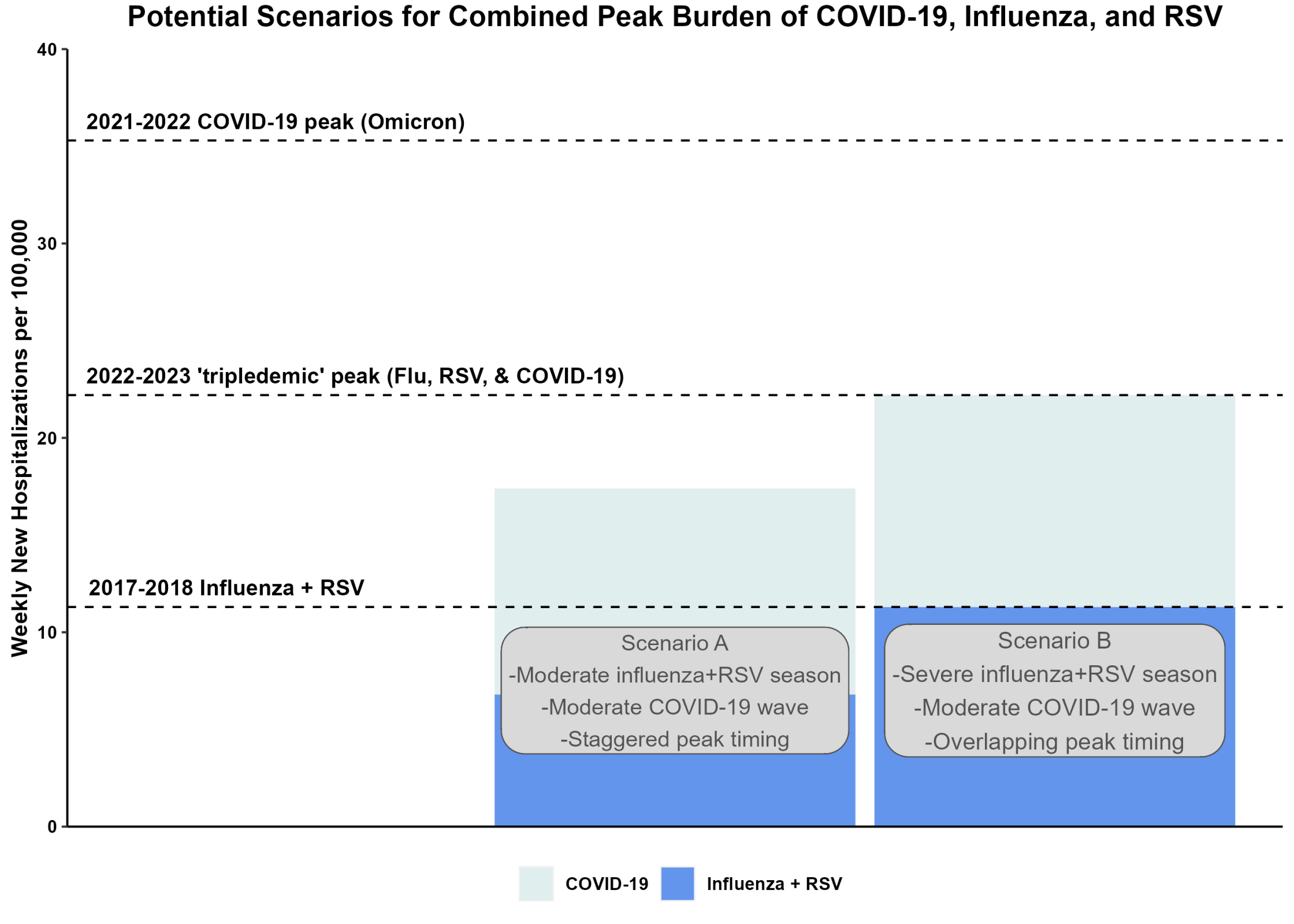
The U.S. Centers for Disease Control and Prevention (CDC) recently reported that fall 2023 respiratory hospitalization 'triple-demic' may not actually occur this year.
On October 26, 2023, the CDC stated it continues to anticipate that the upcoming fall and winter respiratory disease season will likely result in a similar number of hospitalizations as last season.
The CDC wrote .....We continue to have low-to-moderate confidence in this assessment due to uncertainties anticipating the timing and levels of peak disease activity. As of October 20, 2023, respiratory virus activity in the U.S. is low nationally.
Although COVID-19 activity continues to decline in many areas of the United States and globally, it remains the cause of most new respiratory virus hospitalizations and deaths.
Additionally, sustained increases in RSV activity in the southern U.S. indicate the start of the 2023-2024 RSV season, with the mid-Atlantic and Northeastern regions also experiencing elevated activity.
RSV activity remains in line with normal seasonal patterns before the emergence of the SARS-CoV-2 coronavirus.
Furthermore, influenza activity remains low in most areas of the country, but small increases were reported in some places.
According to recent influenza outbreak modeling by the Scenario Modeling Hub, influenza hospitalizations will likely fall within the range observed from 2010 – 2020.
The World Health Organization's (WHO) recent report indicates the CDC's revised forecast is on-target. On October 16, 2023, the WHO published Influenza Update N° 456, showing that influenza and RSV detections remained low globally.
According to the CDC, vaccination remains the best way to protect yourself and your loved ones against serious disease outcomes. As of October 30, 2023, various flu shots and other vaccines are generally available at health clinics and pharmacies in the U.S.
PharmaJet® recently announced the implementation of a study in Nigeria to evaluate the impact of intradermal vaccine administration of fractional inactivated poliovirus vaccine (fIPV) using their Tropis® ID Needle-free Injection System (NFIS).
Announced on October 24, 2023, the study, in collaboration with the National Primary Health Care Development Agency, Jhpiego, PATH, and the Sydani Group, is assessing coverage rates and potential cost reductions associated with using Tropis for fIPV delivery as compared to the current standard of intramuscular delivery of full dose IPV using needle and syringe.
Partners are also evaluating the acceptability and feasibility of using needle-free from the healthcare worker and caregiver perspective.
The study will run through January 2024, with children at 22 urban and rural health facilities receiving fIPV with Tropis. Evidence from the study is intended to inform policy regarding intradermal delivery of polio vaccine in routine immunization settings.
Paul LaBarre, Vice President of Global Business Development, PharmaJet, commented in a press release, “This (effort) builds on our experience in Pakistan where Tropis has been demonstrated to increase coverage rates. Including additional campaigns in Somalia, we have provided more than 10 million syringes for polio immunization programs."
"On this World Polio Day 2023, we restate our commitment to the Global Polio Eradication Initiative.”
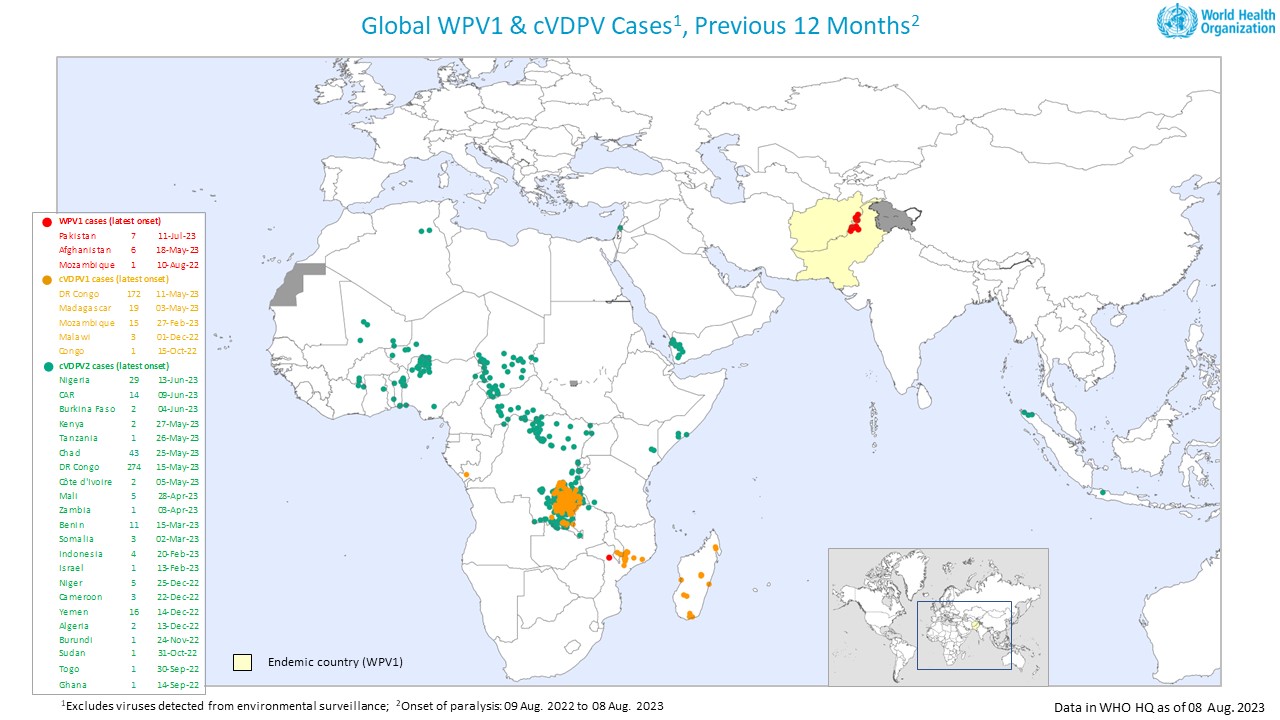
As of October 30, 2023, the U.S. CDC confirms over thirty countries have reported polio outbreaks in the past year.
To mark World Polio Day on October 24, 2023, supporters from over 30 countries joined the Make Polio History campaign to tell governments that eradication is possible and urgently needed.
According to the U.S. Centers for Disease Control and Prevention (CDC), there are thirty-one countries reporting polio cases in 2023.
The CDC says polio is a crippling and potentially deadly disease that affects the nervous system. Some people have only minor symptoms, such as fever, tiredness, nausea, headache, nasal congestion, sore throat, cough, stiffness in the neck and back, and pain in the arms and legs.
In rare cases, polio infection causes permanent loss of muscle function (paralysis). Polio can be fatal if the muscles used for breathing are paralyzed or if there is an infection of the brain.
Countries are deploying an innovative, novel oral polio vaccine type 2 (nOPV2) to better address the evolving risk of type 2 circulating vaccine-derived poliovirus (cVDPV2).
The vaccine is a modified version of the type 2 monovalent OPV vaccine, which clinical trials have shown provides comparable protection against poliovirus while being more genetically stable and less likely to be associated with the emergence of cVDPV2 in low immunity settings.
Thus, nOPV2 can be a significant tool for more sustainably stopping polio outbreaks.
As of October 29, 2023, approximately 820 million doses of nOPV2 had been administered across 35 countries. An additional 16 countries have met the requirements for using nOPV2 in the event of a polio outbreak.
However, the nOPV2 is not authorized by the U.S. Food and Drug Administration.
Since 2000, the only polio vaccine used in the U.S. is the inactivated polio vaccine (IPV), which protects against severe disease, including paralysis. IPVs are generally available at clinics and pharmacies in the U.S.