Search API
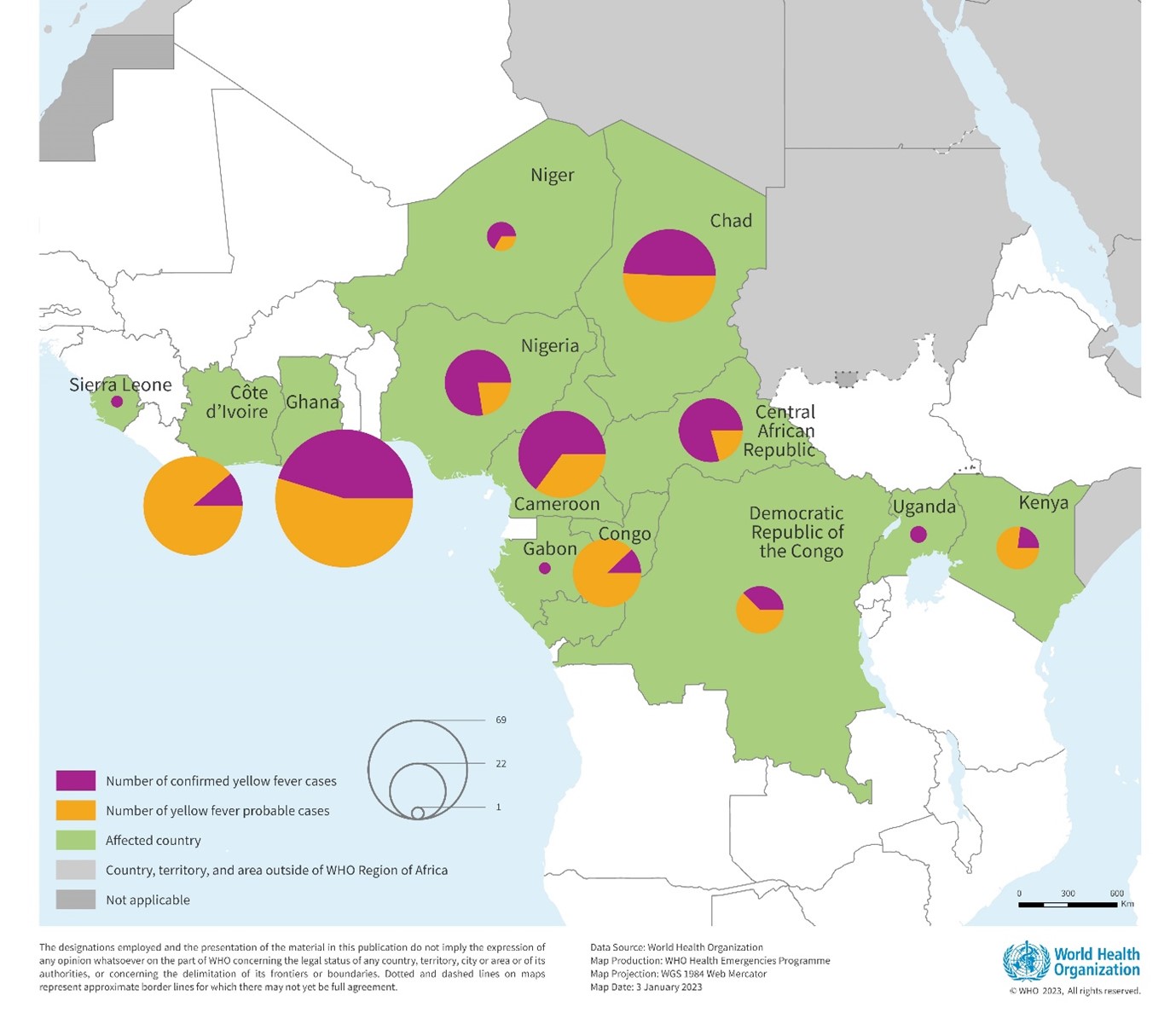
According to an update recently published by the Africa Centres for Disease Control and Prevention (Africa CDC), yellow fever outbreaks continue across the continent in 2023.
As of October 28, 2023, the Africa CDC reported a total of 2,779 yellow fever cases, and 36 deaths (CFR: 1.3%) have been reported in seven African Union countries this year.
The impacted countries are Cameroon (41 cases; 4 deaths), CAR (326; 5), Congo (324; 2), Gabon (79; 0), Guinea (178; 4), Nigeria (1,819; 21), and Uganda (12; 0).
In 2022, 12 countries in the African Region reported confirmed yellow fever cases.
Yellow fever is an epidemic-prone, vaccine-preventable disease transmitted to humans by mosquitoes. The incubation period ranges from 3 to 6 days. While many people do not experience symptoms, individuals can have more severe symptoms.
Death can occur within 7 - 10 days in about half of cases with severe symptoms.
According to the WHO/UNICEF Estimates of National Immunization Coverage in 2021, routine immunization coverage against yellow fever in the African Region for childhood vaccinations was 48%, much lower than the threshold required to confer population immunity.
In reaction to these data, the WHO and Africa CDC re-assessed the health risk at the regional level in 2022 as moderate.
Currently, the U.S. population is mostly unvaccinated against yellow fever, and the U.S. Strategic Stockpile has not secured vaccine reserves.
During a sizable epidemic in the U.S., the demand for yellow fever vaccines could surpass production capacities. Sanofi Pasteur's YF-VAX® vaccine is the only U.S. Food and Drug Administration-approved vaccine as of November 2023.
For many international travelers, proof of yellow fever vaccination is a requirement to visit at-risk countries, such as Brazil.
The Coalition for Epidemic Preparedness Innovations (CEPI) and the University of Oxford announced the launch of a new project to initiate early development of prototype vaccines against the Junín virus.
CEPI confirmed on October 2, 2023, that it would provide up to $25 million to Oxford for preclinical and Phase I clinical development of a vaccine against the Junín virus using Oxford's ChAdOx platform
This seldom-discussed virus was selected as an exemplar of the Arenavirus family, which includes the Lassa virus, Junin virus, Machupo virus, Guanarito virus, and lymphocytic choriomeningitis virus.
Arenavirus infections are responsible for multiple deadly hemorrhagic fevers with epidemic and pandemic potential. Junín virus can cause Argentine Haemmorhagic Fever, with symptoms including muscular pain, dizziness, rashes, and a 15-30% case fatality.
Dr. Richard Hatchett, CEO of CEPI, commented in a press release, "This new project will harness the University of Oxford's extensive vaccinology experience and its innovative ChAdOx vaccine technology – one of only a handful of vaccine platforms proven to work at speed, scale, and low cost – to expand the world's scientific knowledge on arenavirus vaccines."
"The project will generate vital resources for the proposed Global Vaccine Library, helping accelerate efforts to reduce vaccine development timelines to 100 days when faced with future threats."
The data and materials generated by this new project could give the world a head start in rapidly developing safe and effective vaccines against Arenaviruses within 100 days of their identification, potentially stopping a future pandemic in its tracks, wrote CEPI.

Vaxart, Inc. today announced it has dosed the first subject in its Phase 1 clinical trial evaluating Vaxart’s oral pill bivalent norovirus vaccine candidate (VXA-G1.1-NN) focused on lactating mothers.
This new study is evaluating the ability of oral vaccine tablets to induce breast milk antibodies and transfer antibodies to infants.
Norovirus sickens approximately 21 million people in the U.S. annually, including 15% of children under age 5. While pediatric deaths due to norovirus in the U.S. are rare, they are much more common in the developing world.
Currently, there are no U.S. FDA-approved norovirus vaccines.
The Phase 1, multicenter, randomized, double-blind, placebo-controlled, single-dose, dose-ranging VXA-NVV-108 Clinical Trial is designed to evaluate the safety, tolerability, and immunogenicity of orally administered bivalent GI.1/GII.4 norovirus vaccine in healthy lactating females at least 18 years of age and their breast-feeding infants (aged 30 days to 11 months).
The study is expected to enroll approximately 76 subjects at seven sites in South Africa.
“Initiating this study is an important step toward Vaxart’s goal of developing a vaccine that may reduce the significant global health threat norovirus poses to children under five years of age,” said Dr. James F. Cummings, Vaxart’s chief medical officer in a press release on November 2, 2023.
“We believe an oral norovirus vaccine pill may one day allow mothers to protect their infants against this highly contagious virus for which there currently is no approved vaccine.”
The Phase 2b study (VXA-NVV-201) is expected to add safety data that, if successful, could enable Vaxart to schedule an End-of-Phase 2 meeting with the U.S. FDA, potentially in 2024.
Vaxart believes that its proprietary pill vaccine delivery platform is suitable for delivering recombinant vaccines, positioning the company to develop oral versions of currently marketed vaccines and to design recombinant vaccines for new indications.

For the second time in a month, the mosquito-transmitted dengue fever virus has been confirmed in a person in Southern California.
On November 1, 2023, the City of Long Beach Department of Health and Human Services (Health Department) reported one case of dengue in a resident who has not traveled outside the U.S.
This is the first non-travel-related case of dengue in Long Beach, which has reported five travel-related cases.
Long Beach is home to approximately 466,000 Californians.
According to the Health Department, the risk of local exposure remains low.
"The health and well-being of the community is our most important priority," commented Long Beach Mayor Rex Richardson in a press release.
"We are working closely with health officials and doing everything we can to prevent more (dengue) cases. We ask that everyone do their part by removing standing water on their property to help us control the mosquitoes in our neighborhoods."
For more local information, people are encouraged to visit longbeach.gov/dengue.
According to reports, over 4.2 million infections and about 3,000 dengue outbreak-related deaths have been reported from 79 countries/territories in the past year.
In the U.S., the state of Florida has reported the most travel-related and local dengue cases in 2023.
An earlier locally-acquired dengue case was reported in Pasadena, California.
Dengue is a disease that is spread by the bites of Aedes species mosquitoes. When a mosquito bites someone with one of dengue's four viruses in their blood, it can spread the virus to others.
The best way to protect oneself from dengue and other diseases spread by mosquitoes is to avoid mosquito bites.
Dengue vaccines are available in 2023, but there are various geographic limitations and/or testing requirements.

Tonix Pharmaceuticals Holding Corp. today announced that the National Institute of Allergy and Infectious Diseases (NIAID) will conduct a Phase 1 clinical trial with TNX-1800 (recombinant horsepox virus, live vaccine), expected to start in the second half of 2024.
The Phase 1 study involving TNX-1800 assesses safety and immunogenicity in approximately 60 healthy adult volunteers.
Tonix is developing a novel vaccine platform that, primarily by eliciting a T-cell immune response, will provide durable protection against severe disease and prevent forward transmission.
A related horsebox-based vaccine, TNX-8011, protected animals against challenge with monkeypox virus delivered directly into the lungs.
TNX-801 is also the vector on which TNX-1800 is based and has been shown to be >1,000-fold more attenuated than modern vaccinia virus vaccine (VACV) strains in immunocompromised mice.
Seth Lederman, M.D., CEO of Tonix Pharmaceuticals, commented in a press release on November 2, 2023, "TNX-1800 will be the first vaccine candidate using our live virus recombinant pox virus (RPV) platform technology to enter clinical trials."
"We hope to expand the portfolio of RPV-based vaccines to address several other known respiratory threats, including smallpox, mpox and tuberculosis."
"We are committed to supporting NIAID in assembling a variety of vaccine platform options to ensure the availability of effective vaccines in the face of known and emerging threats."
"We look forward to participating in the Project NextGen initiative."
Project NextGen is an initiative by the U.S. Department of Health and Human Services to advance a pipeline of new, innovative vaccines and therapeutics.
As of November 2023, mpox vaccines are U.S. FDA-approved and available in certain cities.

Novavax, Inc. today announced the European Commission has approved the Nuvaxovid™ XBB.1.5 COVID-19 vaccine for individuals aged 12 and older.
This decision follows a positive opinion on approval from the European Medicines Agency's Committee for Medicinal Products for Human Use.
Novavax is working closely with EU member states that have requested doses through the advance purchase agreement to confirm timing of dose delivery on a country-by-country basis.
Novavax's vaccine is authorized for use in the U.S.
"Today's approval of the only updated protein-based non-mRNA COVID-19 vaccine in the EU is an important milestone as the need for vaccination continues," said John C. Jacobs, President and Chief Executive Officer, Novavax, in a press release on October 31, 2023.
"Novavax is working closely with national authorities to have our updated vaccine delivered and available in Europe in the coming weeks."
Nuvaxovid™ XBB.1.5 (recombinant, adjuvanted) (NVX-CoV2601) approval was based on non-clinical data showing that Novavax's updated COVID-19 vaccine induced functional immune responses against XBB.1.5, XBB.1.16 and XBB.2.3 variants.
Additional non-clinical data demonstrated that Novavax's vaccine-induced neutralizing antibody responses to newly emerging subvariants BA.2.86, EG.5.1 FL.1.5.1 and XBB.1.16.6 as well as CD4+ polyfunctional cellular (T-cell) responses against EG.5.1 and XBB.1.16.6.
These data indicate that Novavax's vaccine can stimulate both arms of the immune system and may induce a broad response against circulating variants.1,2
In clinical trials, the most common adverse reactions to Novavax's prototype COVID-19 vaccine (NVX-CoV2373) were headache, nausea or vomiting, muscle pain, joint pain, injection site tenderness, injection site pain, fatigue, and malaise.
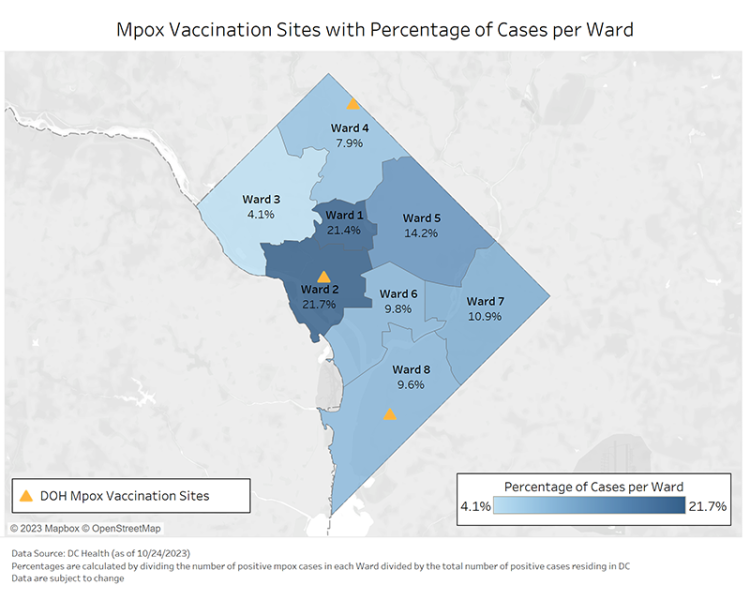
According to a study published in Sexually Transmitted Diseases, most study participants receiving mpox vaccination in Washington, DC, reported decreasing sexual behaviors associated with mpox virus transmission.
Overall, between 46%–61% of study participants reported a decrease in sexual behaviors associated with mpox.
In DC, over 41,000 mpox vaccinations have been administered since the global outbreak began in May 2022.
Published on October 26, 2023, the study was led by U.S. Centers for Disease Control and Prevention researchers and involved 711 adults seeking mpox vaccination from August to October 2022.
The median participant age was 32 years; 52% were White, 20.5% were Black, 14.6% were Hispanic, 7.9% were Asian, 2.0% were multiracial, and 0.3% were American Indian/Alaska Native.
And 9% had HIV.
Most of the study participants were men who had sex with men (61%), 27% were women, and 3.8% were men who had sex with only women.
According to D.C. Health, there have been 543 cumulative mpox cases, 24 hospitalizations, and 0 related fatalities since May 2022.
Mpox is a sexually transmitted disease that is often prevented with a U.S. FDA-approved vaccine.
Bavarian Nordic's JYNNEOS® (MVA-BN®, IMVAMUNE®) mpox-smallpox vaccine was offered in the U.S. during this study.
A study published by The Lancet Infectious Disease on October 11, 2023, attempted to answer questions regarding the durability and strength of protection following infection and JYNNEOS vaccination.
This analysis reported that people vaccinated with JYNNEOS frequently developed low or medium mpox-neutralizing antibodies compared to infected individuals.
As of October 31, 2023, the JYNNEOS vaccine remains available in key cities in the U.S.