Search API
In a Lancet Respiratory Medicine news article published on November 6, 2023, Sean O'Leary, MD, chair of the American Academy of Pediatrics (AAP)'s Committee on Infectious Diseases, stated the nationwide shortage of Beyfortus™ (Nirsevimab-alip), a newly approved respiratory syncytial virus (RSV) monoclonal antibody, could have been predicted.
"I would've predicted pretty high demand. I think probably too much was made of vaccine hesitancy and refusal..." wrote Dr. OLeary.
Sanofi, the producer of Beyfortus, stated on October 26, 2023, 'Despite an aggressive supply plan built to outperform past pediatric immunization launches, demand for this product, especially for the 100 mg doses used primarily for babies born before the RSV season, has been higher than anticipated.'
Sanofi collaborates closely with the U.S. Centers for Disease Control and Prevention (CDC) to ensure equitable distribution of available doses through the Vaccines for Children Program.
The CDC recently issued an advisory with recommendations for clinicians to prioritize 100-milligram doses for infants younger than six months and those with underlying medical conditions that predispose them to severe RSV.
Beyfortus is the second monoclonal antibody developed to prevent RSV in young children.
The AAP has recommended Arexis AB's palivizumab (Synagis) for high-risk infants and young children during an active RSV season.
Synagis was approved for initial use in the U.S. by the FDA in 1998. It is not an RSV vaccine but can help passively protect children with monthly dosing.
As of November 7, 2023, the RSV season began in Florida and has spread throughout the United States, impacting certain areas.

Clover Biopharmaceuticals, Ltd. announced today that it has completed the Biologic License Application (BLA) submission for its seasonal influenza vaccine (AdimFlu-S) to the Brazilian Health Regulatory Agency.
Upon approval, Clover will work with its local partner to commercialize AdimFlu-S, a quadrivalent split-inactivated vaccine containing hemagglutinin from four influenza virus strains (A and B).
If approved in Brazil, Clover's AdimFlu-S would have access to the Southern Hemisphere market.
Brazil is a vital vaccine market strategically. The country has the world's second-largest seasonal influenza vaccine market. Its market size is expected to surpass US$1 billion over the next five years.
"The BLA submission of AdimFlu-S in Brazil is another step towards our goal of becoming a global leader in the respiratory vaccine space and builds upon Clover's prior experience enrolling over 10,000 people in clinical trials across Brazil and South America," said Joshua Liang, Chief Executive Officer, and Executive Director of Clover, in a press release on November 6, 2023.
"By leveraging our unique globalization capabilities, we will continue expanding to other countries and regions to diversify our sales and maximize our impact on public health."
As of September 2023, AdimFlu-S has been listed in 26 provinces and municipalities in China.
Clover's diverse pipeline of candidates includes potential treatments that could significantly reduce the burden of vaccine-preventable diseases and make more diseases preventable.
In the United States, 145.42 million influenza vaccine doses (egg-based, nasal, cell-based) had been distributed in the U.S. as of October 28, 2023,

The World Health Organization's (WHO) 2023 Global Tuberculosis (TB) report, announced today, shows the impact of this centuries-old disease.
The report, published on November 7, 2023, features TB outbreak data from 192 countries and areas and shows that 7.5 million people were diagnosed in 2022, the highest figure recorded since 1995.
According to the WHO, an estimated 10.6 million people fell ill with TB in 2022, up from 10.3 million in 2021.
And the total number of TB-related deaths (including those among people with HIV) was 1.3 million in 2022. TB continues to be the leading killer among people with HIV.
Geographically, most people who developed TB in 2022 were in South-East Asia (46%), Africa (23%), and the Western Pacific (18%), with smaller proportions in the Eastern Mediterranean (8.1%), the Americas (3.1%) and Europe (2.2%).
In a press release, Dr. Tereza Kasaeva, Director of WHO's Global TB Programme, commented, "This report provides key data and evidence on the status of the TB epidemic and a review of the progress that serves to inform the translation of these targets and commitments into action in countries."
"We need all hands on deck to make the vision of ending TB a reality."
TB is a vaccine-preventable disease, with about 16 different Bacille Calmette-Guérin (BCG) vaccines in use globally.
In the U.S., access to the BCG vaccine is limited and considered for people who meet specific criteria. Merck's TICE® BCG vaccine is an attenuated, live culture preparation of the BCG strain of Mycobacterium Bovis and is available in 2023.

The World Health Organization (WHO) recently announced the Health Sciences Authority (HSA), Singapore; the Ministry of Food and Drug Safety (MFDS), Republic of Korea; and the Swiss Agency for Therapeutic Products (Swissmedic), Switzerland are the first three countries to be listed as WHO-Listed Authorities.
A WHO-listed authority (WLA) is a regulatory authority or a regional regulatory system that has been documented as complying with all the indicators and requirements specified by the WHO for the requested scope of listing based on an established benchmarking and performance evaluation process.
“This achievement is the result of investment by the Governments of the Republic of Korea, Singapore, and Switzerland in the strengthening of their regulatory systems and reaffirms the collaboration between WHO and the three Governments in promoting confidence, trust, and further reliance on authorities that have attained this global recognition, through the transparent and evidence-based pathway for designating and listing of WLAs,” said Dr. Yukiko Nakatani Assistant Director-General for Access to Medicines and Health Products, in a press release on October 31, 2023.
Implementing the WLA framework is intended to promote access to and supply of safe, effective, and quality medical products.
It is expected that HSA, MFDS, and Swissmedic will sustain this achievement, thereby enabling greater regulatory efficiencies and more informed decision-making at the national, regional, and global levels, wrote the WHO.

GC Biopharma announced today that it has applied to the Korean Ministry of Food and Drug Safety for the marketing approval of "GC1109", an anthrax vaccine jointly developed by the company and the Korea Disease Control and Prevention Agency (KDCA).
If approved, "GC1109" will be the world's first recombinant anthrax vaccine.
'GC1109' contains a protective antigen as its active pharmaceutical ingredient, produced by recombinant DNA technology. This delivers two types of proteins, comprising anthrax toxin, lethal factor, and edema factor, into cells.
In the Phase II clinical trial, the vaccine's immunogenicity and safety were demonstrated with healthy volunteers. Subjects who received intramuscular administration of GC1109 generated antibodies sufficient to neutralize anthrax toxins, while adverse drug reactions or solicited adverse events were similar to those of the placebo group.
In the animal efficacy study, GC1109 induced neutralizing antibody, which remained at a high level even six months after the 4th dose of the vaccine, with a high survival rate against the bacillus anthracis spore challenge.
GC Biopharma stated in a press release on November 5, 2023, "We believe in the significance of our journey to localize the anthrax vaccine in terms of securing vaccine sovereignty while promoting public health and national security."
"GC Biopharma will continue to lead the localization of critical medicines and contribute to the stable supply of basic medical supplies as it has been doing for other vaccines and blood products since the foundation of the company."
Since it is unethical to expose human volunteers to lethal Bacillus anthracis, and field trials are not feasible due to a low incidence of anthrax, human clinical efficacy studies for an anthrax vaccine cannot be conducted.
In such cases, under the "Animal Rule" of The Special Act for Promotion of the Development and Emergency Supply of Medical Products in Response to Public Health Crisis, animal efficacy data can be used to establish the vaccine's clinical benefit if the evidence from the animal studies provides substantial grounds for the product's effectiveness.
Anthrax, caused by Bacillus anthracis, is a Class 1 infectious disease by the Infectious Disease Control and Prevention Act, with a lethality rate of up to 97% if untreated.
To prepare against potential bioterrorism and consequent national crisis, GC Biopharma, under the research project supported by KDCA, has been developing a recombinant vaccine for anthrax since 2002.
GC Biopharma (formerly Green Cross Corporation) is a biopharmaceutical company that delivers life-saving and life-sustaining protein therapeutics and vaccines.
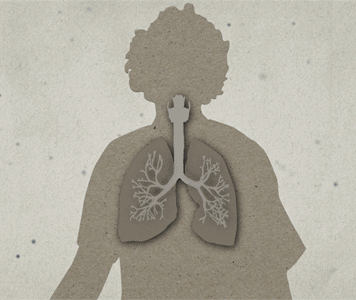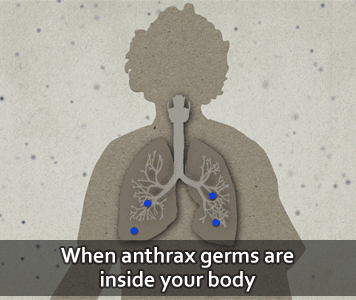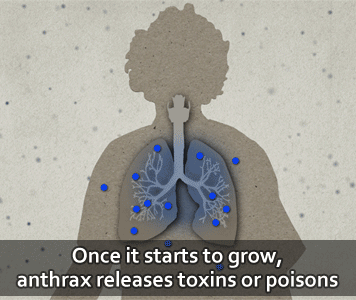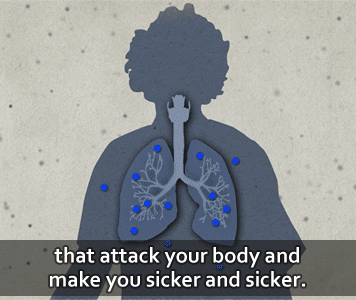
According to the U.S. CDC, people get infected with anthrax when spores get into the body. When this happens, the spores can be activated and become anthrax bacteria.
Then, the bacteria can multiply, spread out in the body, produce toxins (poisons), and cause severe illness.
The good news is anthrax is not contagious.
You cannot catch anthrax from another person the way you might catch a cold or the flu.
In rare cases, person-to-person transmission has been reported with cutaneous anthrax, where discharges from skin lesions might be infectious, says the CDC.
Moffitt Cancer Center today announced researchers are working to improve the efficacy of neoantigen-targeted cancer vaccines by better understanding whether primary or metastatic tumors should be used to produce the personalized vaccine.
On November 5, 2023, these cancer specialists launched a study evaluating primary and metastatic tumor pairs from 45 patients with several solid tumor types, including melanoma, bladder, head and neck cancers, and non-small cell lung cancer.
Results presented at the Society for Immunotherapy of Cancer annual meeting show that melanoma, bladder, and head and neck tumors share a high percentage of mutations between primary and metastatic tumors.
However, other solid tumors, such as esophageal and non-small cell lung cancer, share less.
Whole exome sequencing was used to identify somatic alterations, which are genetic mutations or DNA alterations that may impact the type of antigens produced by the cancer cells that the vaccine can then target.
Dr. Ahmad Tarhini, Director, Cutaneous Clinical and Translational Research at Moffitt, commented in a press release, "Our analysis demonstrates genetic variations that exist when comparing paired primary and metastatic tumors that appear to vary by histology."
"Variants are potentially undergoing negative selection supported by the preferential loss of out-of-frame events in metastatic tumors."
Understanding the clonal structure will be vital to predicting neoantigens for effective neoantigen-based vaccines, where oncogenic drivers can be prioritized and used to determine the primary clones.
Tarhini and the Moffitt team continue this work, expanding their study to include paired tumor samples from 600 additional patients.
As Florida's only National Cancer Institute-designated comprehensive cancer center and one of only 30 leading cancer centers in the U.S. participating in the National Comprehensive Cancer Network, Moffitt is at the forefront of cancer centers worldwide.

The U.S. Food and Drug Administration (FDA) MedWatch Safety Alert recently published an advisory on X informing healthcare providers who administer the Moderna COVID-19 vaccine (SpikeVax® XBB.1.5) (2023-2024 Formula) to individuals six months through 11 years of age to ensure that the correct volume of the vaccine (0.25 mL) is withdrawn from the vial and that the correct dose is administered to the vaccine recipient.
On November 1, 2023, the FDA confirmed that it had become aware that some healthcare providers may not recognize that the single-dose vial of Moderna COVID-19 Vaccine (2023-2024 Formula) for use in individuals six months through 11 years of age contains notably more than 0.25 mL of the vaccine.
Some healthcare providers may be withdrawing the entire contents of the vial to administer to an individual.
The Dosage and Administration section of the Fact Sheet for Healthcare Providers Administering Vaccine has been revised to clarify that 0.25 mL should be withdrawn from the vial, and the vial and any excess volume should be discarded.
If healthcare providers, parents, or caregivers have questions, they may contact the FDA's Center for Biologics Evaluation and Research at [email protected].
On November 2, 2023, Moderna, Inc. reported financial results and provided business updates for the third quarter of 2023, but these did not highlight this MedWatch advisory.
"Through this quarter, we demonstrated our ability to increase our share in the U.S. market (Spikevax's U.S. market share to date increased to 45% from 36% in 2022). We now expect this year's vaccination rate to be similar to last fall," commented Stéphane Bancel, Moderna's CEO, in a press release.
"In the third quarter, we significantly resized our manufacturing infrastructure to make our COVID-19 franchise profitable for 2024 and beyond.
The Company reported $1.8 billion in Spikevax® vaccine sales in the third quarter of 2023. This led to $3.9 billion in vaccine sales for the year through the third quarter.
Moderna believes that the U.S. market for fall 2023 will be at least 50 million doses, supporting total 2023 Spikevax sales of at least $6 billion.