Search API
The U.K.'s Joint Committee on Vaccination and Immunisation (JCVI) today advised the government of a new, routine targeted vaccination program to prevent gonorrhea.
On November 10, 2023, the JCVI announced it had agreed that a targeted program should be initiated using the 4CMenB (Bexsero®) vaccine for the prevention of gonorrhea in those who are at most significant risk of infection.
The JCVI advice is that Bexsero should be offered on an opportunistic basis through specialist sexual health services, which have the experience in assessment and identification of those who are at increased risk of infection with bacterial sexually transmitted infections (STI).
The U.S. Food and Drug Administration (FDA) initially approved Bexsero (Meningococcal Group B Vaccine) for intramuscular injection in 2015.
In the U.S., Bexsero is generally available at health clinics and pharmacies.
As of November 2023, no vaccines are approved by the FDA or European Medicines Agency for preventing gonorrhea infection.
The U.S. Centers for Disease Control and Prevention (CDC) reported about 710,000 cases of Gonorrhea in 2021, making it the second most common STI in the U.S.
In addition, the CDC announced on April 11, 2023, that gonorrhea rates increased by more than 4%, reaching 710,151 in 2022.

According to a new U.S. CDC Morbidity and Mortality Weekly Report (MMWR), during the 2022–23 influenza season, among approximately 8.4 million healthcare providers (HCPs) working in over 4,000 acute care hospitals, influenza vaccination coverage was 81% at acute care hospitals and 47% among those at nursing homes.
Published on November 10, 2023, this MMWR confirmed influenza vaccination coverage was highest in the Pacific region (61.1%) and lowest in the South (39.7%).
These CDC researchers wrote, 'Implementing vaccination recommendations for HCP has been a long-standing challenge for the public health and health care sectors.'
'In an effort to improve vaccination coverage among HCP, health care facilities, and federal and state governments have implemented interventions including jurisdiction-wide and facility-wide vaccination mandates.'
The CDC continues to recommend vaccination as long as flu viruses pose a threat in the U.S. During some flu seasons, that can be as late as May or June.

According to the U.S. Centers for Disease Control and Prevention (CDC), during the 2022–23 school year, nationwide vaccination coverage among kindergarten children was about 93% for common vaccines, similar to that in the 2021–22 school year.
However, last year's data was lower than the 94% coverage in the 2020–21 school year and lower still than the 95% coverage during the 2019–20 school year.
On November 10, 2023, the CDC announced two concerning trends.
The overall percentage of children with an exemption increased from 2.6% during the 2021–22 school year to 3.0% during the 2022–23 school year, the highest exemption rate ever reported in the United States.
Overall, the percentage of children with a vaccine exemption increased in 40 states and D.C.
Nonmedical exemptions account for about 90% of reported exemptions and approximately 100% of the increase in the national exemption rate.
Furthermore, from the 2019–20 to the 2021–22 school year, national coverage with state-required vaccines among kindergartners declined from 95% to approximately 93%, ranging from 92.7% for DTaP to 93.1% for polio.
And the National measles, mumps, and rubella (MMR) coverage among kindergarten students remained below the Healthy People 2030 target of 95% for the third consecutive year.
Nationally, 2-dose MMR coverage was 93.1% (range = 81.3% [Idaho] to ≥98.4% [Mississippi]).
While there have been a few measles outbreaks in the U.S. this year, several countries, such as India, continue reporting significant measles outbreaks in late 2023.
Various MMR vaccines remain available at most clinics and pharmacies in the U.S. A January 2023 IQVIA study showed that 60-70% of all flu vaccines are administered at pharmacies during late 2022.
To address the declines in routine immunization coverage across the lifespan, the CDC launched the Let's Routine Immunizations on Schedule for Everyone (RISE) initiative earlier in 2023 and provides a broad range of communication and enhanced technical assistance, including back-to-school campaigns.

Valneva SE today announced that the U.S. Food and Drug Administration (FDA) approved IXCHIQ®, the Company’s single-dose, live-attenuated vaccine indicated for the prevention of disease caused by chikungunya virus (CHIKV) in individuals 18 years of age and older who are at increased risk of exposure to CHIKV.
This indication is approved under accelerated approval based on anti-CHIKV neutralizing antibody titers. Valneva had received a Priority Review Voucher from the FDA.
“Infection with chikungunya virus can lead to severe disease and prolonged health problems, particularly for older adults and individuals with underlying medical conditions,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, in a press release on November 9, 2023.
“Today’s approval addresses an unmet medical need and is an important advancement in preventing a potentially debilitating disease with limited treatment options.”
Valneva reported final pivotal Phase 3 data for the vaccine in March 2022, showing a 98.9% seroresponse rate at 28 days with a single vaccination and final lot-to-lot consistency results in May 2022.
IXCHIQ-induced seroresponse was sustained over time with a 96.3% seroresponse rate six months post-vaccination.
The Company’s pivotal Phase 3 results were published in the Lancet in June 2023.
Valneva will continue to evaluate antibody persistence for at least five years. Continued approval for this indication is contingent upon verification of clinical benefit in confirmatory studies.
With this U.S. approval, IXCHIQ becomes the world’s first licensed chikungunya vaccine available to address this unmet medical need and the third vaccine Valneva has brought from early R&D to approval.
The Company will hold an analyst call and a webcast at 3:00 pm CET or 9:00 am EDT on November 13, 2023. The link will be available on the Company’s investor page.
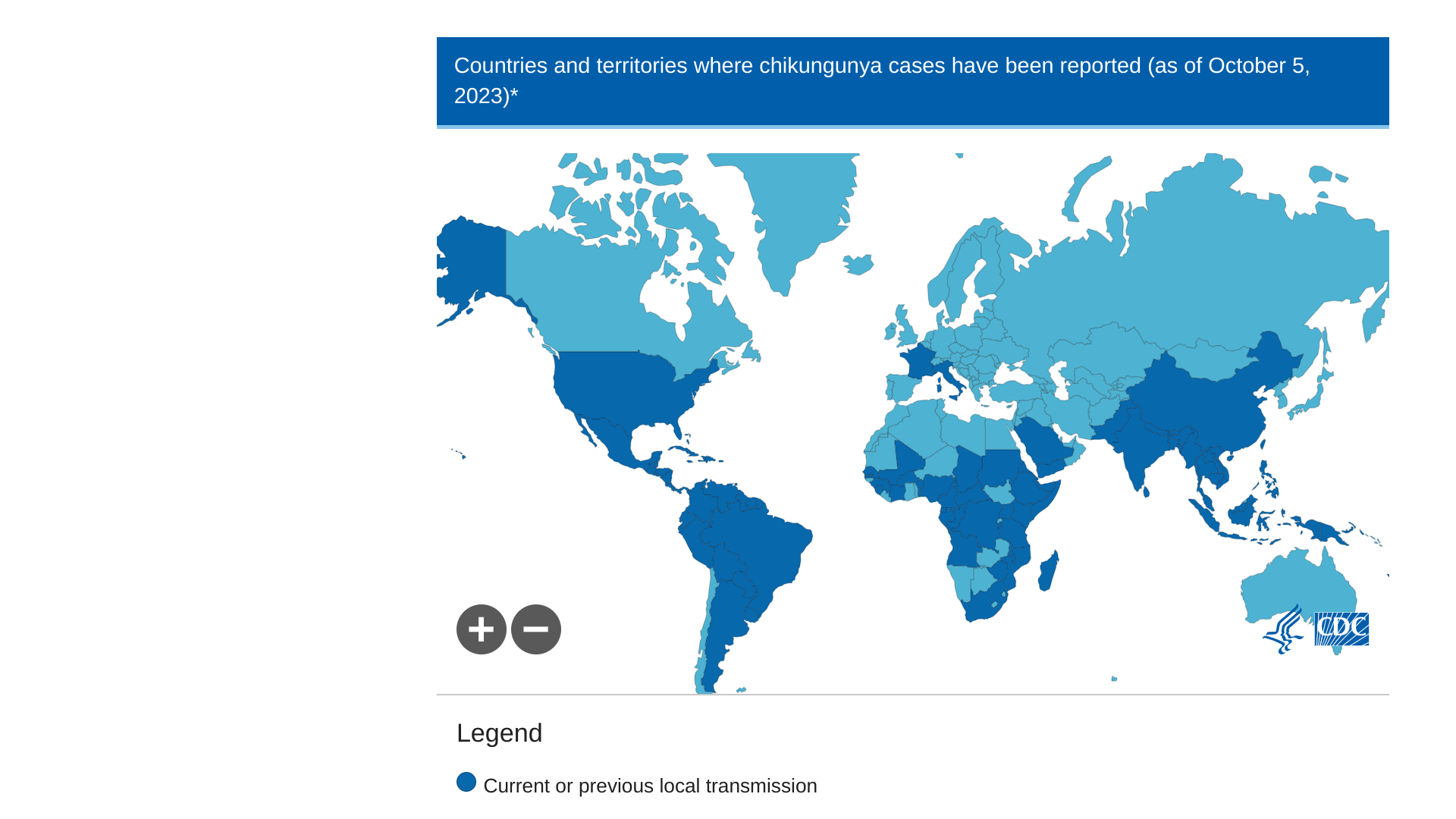
Chikungunya outbreaks are primarily found in Africa, the Americas, Asia, and the Indian subcontinent. CHIKV outbreaks are related to other vaccine-preventable travel diseases, such as dengue, measles, and polio.
The U.S. CDC published Recommendations for Chikungunya Vaccine Use Among Adult Travelers on October 26, 2023.
Vaxcyte, Inc. today announced that the first participants were dosed in a Phase 1/2 clinical study for VAX-31, a 31-valent pneumococcal conjugate vaccine (PCV) candidate designed to prevent invasive pneumococcal disease (IPD) in adults.
Vaxcyte stated it expects to announce topline data from the Phase 1/2 study in the second half of 2024.
"We are pleased to initiate the first adult clinical study of VAX-31, which is the broadest-spectrum PCV to enter the clinic, with the potential to protect against approximately 95 percent of IPD circulating in the U.S. adult population," said Grant Pickering, Chief Executive Officer and Co-founder of Vaxcyte, in a press release on November 9, 2023.
"Leveraging the foundation established by our lead PCV candidate, VAX-24, we believe we have an opportunity to deliver a best-in-class PCV franchise that provides the broadest spectrum of coverage and improved immune responses compared to the standard-of-care in adults today."
The VAX-31 Phase 1/2 clinical study is a randomized, observer-blind, active-controlled, dose-finding clinical study designed to evaluate the safety, tolerability, and immunogenicity of VAX-31 compared to Prevnar 20® in healthy adults aged 50 and older.
Pneumococcal disease (PD) is an infection caused by Streptococcus pneumoniae bacteria. It can result in IPD, including meningitis and bacteremia, and non-invasive PD, including pneumonia, otitis media, and sinusitis.
The U.S. National Center for Health Statistics Mortality Surveillance reported on October 27, 2023, that most respiratory disease deaths were recently related to pneumonia, not COVID-19 or influenza. Precision Vax posts additional PCV vaccine and candidate news.
The U.S. CDC's pneumococcal vaccine schedules were updated in 2023.

Brazil's Ministry of Agriculture and Livestock today announced it had extended the declaration of an animal health emergency for 180 more days across the country due to the identification of outbreaks of the highly pathogenic H5N1 avian influenza virus, mainly in wild birds.
This measure enables the implementation of preventive policies to protect commercial birds from contamination by the disease, also known as avian influenza.
Brazil initially declared an animal health emergency on May 22, 2023, a week after the first detection of contaminated migratory wild birds. Within six months, 139 outbreaks have been identified.
According to the World Organization for Animal Health protocol, as there have been no cases of commercial birds, Brazil maintains its status as an H5N1-free country.
Due to the virus's high mutation and adaptability capacity to new hosts, H5N1 represents a risk mainly to international trade in poultry products. It occasionally poses a threat to human and animal health. Brazil holds a 35% share of the global chicken meat market and is the world's largest exporter.
Although no human disease cases have been reported in Brazil, data from the Pan American Health Organization in 2023 indicate cases in the Americas, including the United States, Chile, and Ecuador.
As of November 9, 2023, bird flu outbreak news is posted by Precision Vax.
Furthermore, the U.S. FDA has approved various avian influenza vaccines since 2013, with newer bird flu vaccine versions approved in 2023.

The Lancet Oncology recently published a Commentary by Hitt Sharma and colleagues reporting findings from a pivotal phase 2/3 human papillomavirus (HPV) prophylactic vaccine trial.
Their article, published on November 7, 2023, showed that two doses of a quadrivalent virus-like particle (VLP) vaccine targeting HPV types 6, 11, 16, and 18 (CERVAVAC®; Serum Institute of India) induced non-inferior antibody responses in girls and boys aged 9–14 years compared with a quadrivalent HPV vaccine licensed worldwide and targeting the same HPV types (Gardasil®) given in three doses to women aged 15–26 years, an age group in which strong clinical efficacy has been shown.
The authors also reported no significant differences in adverse events between the vaccines.
It is the first vaccine manufactured in India to receive licensure by the Drugs Controller General of India.
As of November 9, 2023, there are several HPV vaccines licensed globally.
In the U.S., HPV vaccinations for women began in 2006 and for men in 2011. On August 25, 2023, the U.S. CDC reported that in 2022, 76% of people aged 13–17 had received one or more HPV vaccine doses.
The current U.S. CDC HPV schedule was updated in 2023.
A study led by Chicago Department of Public Health researchers published in the Journal of Infectious Diseases involved estimating rates of HIV, gonorrhea, and chlamydia among mpox patients.
This study was published on November 8, 2023, and identified factors related to mpox severity from June 2022 to March 2023.
These researchers concluded that sexually transmitted infections (STIs) could facilitate mpox transmission.
Of the 1,124 mpox patients, 44% had HIV, and 70% had a previous or current STI, with 39% having had at least three previous STI episodes.
Of 335 vaccinated mpox patients, 55% had received one dose of the JYNNEOS® (MVA-BN) vaccine, and 45% had received two doses.
In total, 17.6% has received one or more JYNNEOS vaccination before mpox infection.
The U.S. Centers for Disease Control and Prevention (CDC) reported on October 25, 2023, that post-JYNNEOS vaccination reinfection cases have been published and that they are aware of less than 10 cases of probable reinfection.
The CDC reported Vaccine Effectiveness of JYNNEOS against mpox ranges from 36%–75% for 1-dose vaccination and 66%–89% for 2-dose vaccination.
"Future research should examine predictors of mpox infection among those with STIs, including other STIs, such as syphilis, HIV risk at STI screening or anatomical site of infection," wrote these researchers.
As of October 2023, there has been a significant increase in mpox outbreaks in the European Region.
In the last month, 21 countries reported mpox cases, with Portugal reporting the highest relative increase in cases (n = 86).

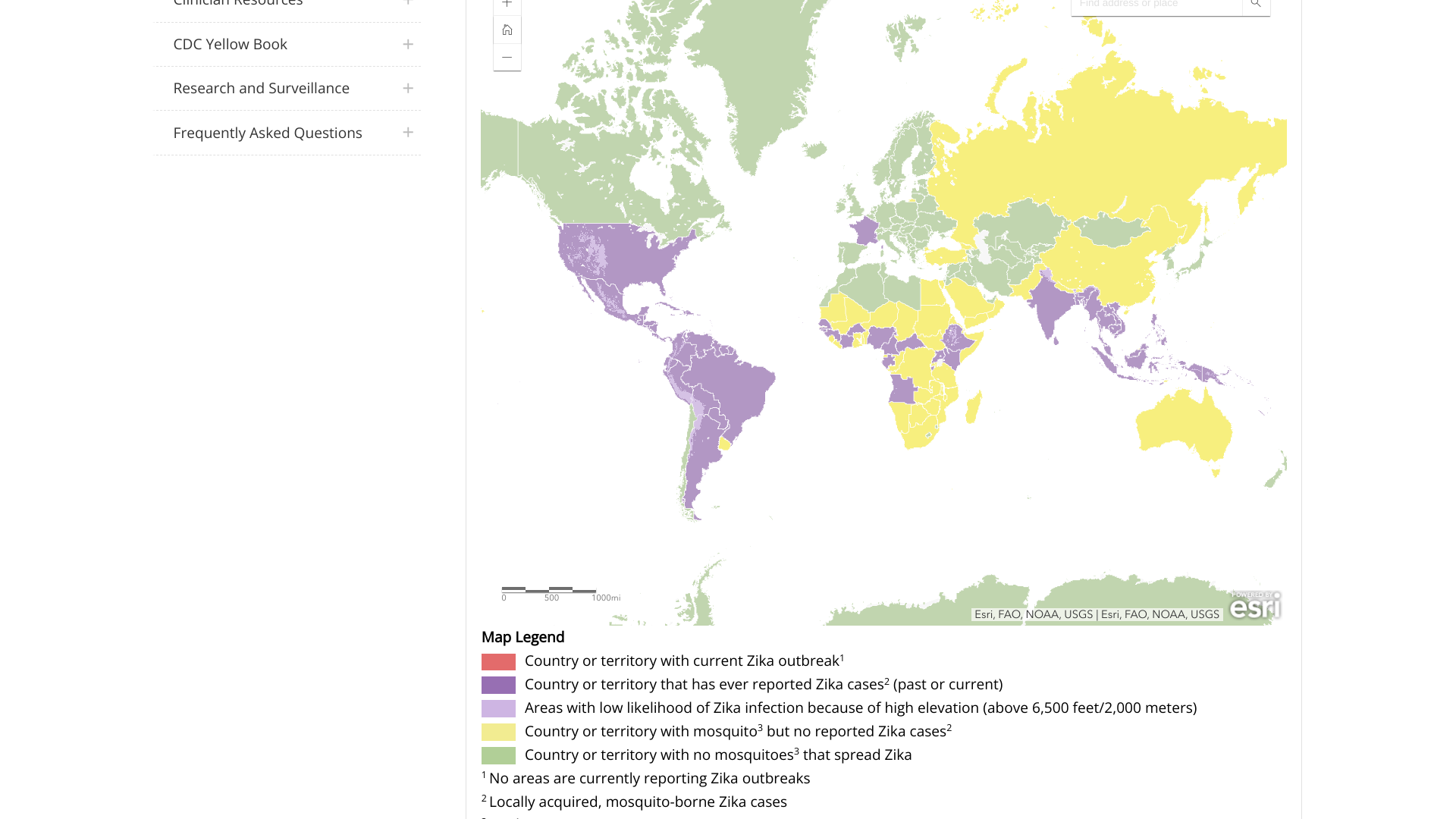
While most people assume Zika virus outbreaks ended a few years ago, new data from the Pan American Health Organization (PAHO) clearly indicates that Zika remains a significant health risk.
As of November 8, 2023, the PAHO dashboard reported 28,267 Zika cases from countries in the Americas, primarily in Central and South America.
The leading countries reporting Zika outbreaks are led by Brazil, with over 26,000 cases, followed by Bolivia, Belize, Columbia, Paraguay, and Venezuela.
In the United States, Puerto Rico's Weekly Report on Arboviral Diseases shows 33 probable Zika cases this year and 20 cases in 2022.
Zika is primarily spread to people by the bite of a mosquito infected with the virus, but it can also be passed during sex from a person infected with Zika.
Furthermore, Congenital Zika-associated syndrome is a set of anomalies (microcephaly) seen in infants born to mothers with a history of gestational Zika fever.
The U.S. CDC recommends that pregnant women and couples planning a pregnancy within the next three months consult with a healthcare provider before visiting an area reporting a Zika outbreak.
As of November 2023, there are no approved Zika vaccines.