Search API
Prof Mojisola Christianah Adeyeye, Director-General of the Federal Republic of Nogeria's National Agency for Food and Drug Administration And Control (NAFDAC), today announced it granted registration approval for the R21/Matrix-M™ vaccine.
The NAFDAC approval on April 17, 2023, is essential since the WHO African Region continues to carry a disproportionately high share of the global malaria burden.
For example, in 2021, the Region was home to about 95% of all malaria cases and 96% of deaths.
And malaria is transmitted throughout Nigeria, with 97% of the population at risk of malaria.
Furthermore, the prevalence of malaria parasitemia in Nigerian children under five is about 23%.
The R21 was created by the University of Oxford Jenner Institute in England, is manufactured by Serum Institute of India Pvt. Ltd., and includes Novavax AB proprietary saponin-based Matrix-M adjuvant.
In addition to malaria, the U.S. CDC has issued various Travel Advisories regarding disease outbreaks in Nigeria.
Vaccine-preventable diseases such as yellow fever, measles, and polio are health risks when visiting Nigeria in 2023.
INOVIO today announced that Dr. Angela Huttner, Infectious Disease Consultant, Geneva University Hospitals presented data from a Phase 1b trial evaluating INO-4201, an Ebola booster vaccine candidate for rVSV-ZEBOV (Ervebo®).
"Preliminary data showed that INO-4201 was well tolerated and produced a strong immune response," stated Dr. Huttner in a press release on April 17, 2023.
"This suggests that a booster dose of IN0-4201 has the potential to extend protection against Ebola and could be an important tool in future Ebola Virus Disease prevention."
In February 2023, INOVIO announced positive initial results from the Phase 1b trial that evaluated INO-4201 as a booster in healthy adult participants who previously received a single injection of Ervebo.
These initial results showed that INO-4201 was well-tolerated and boosted humoral responses in 100% (36 of 36) of treated participants. In addition, data presented today included the assessment of binding antibodies showing that all 36 vaccine recipients responded to the boost.
The unedited press release is posted at this link.
Note: Merck's Ervebo® Vaccine is a live, recombinant, replication-competent vaccine approved by the U.S. FDA and by the European Medicines Agency.
Since 2019, approximately 300,000 persons have been vaccinated with the Ervebo vaccine in Africa.
Other Ebolavirus (Zaire and Sudan) vaccine and outbreak(s) news is posted at Vax-Before-Travel.
Vaxcyte, Inc. today announced positive results from the VAX-24 Phase 2 study in adults aged 65 and older, as well as data from the full six-month safety assessment and prespecified pooled immunogenicity analyses from both the Phase 2 study in adults aged 65 and older and the prior Phase 1/2 study in adults aged 18-64.
VAX-24, the Company's lead, broad-spectrum 24-valent pneumococcal conjugate vaccine (PCV) candidate, is being studied to prevent invasive pneumococcal disease (IPD).
The Company says, 'The public health community continues to affirm the need for vaccines that offer broader protection to prevent IPD.'
"Based on the overall strength of our data and the well-established regulatory pathway, we look forward to meeting with regulators and advancing VAX-24 into a pivotal Phase 3 study for which we expect topline data in 2025," said Grant Pickering, Chief Executive Officer and Co-Founder of Vaxcyte, in a press release on April 17, 2023.
"We developed VAX-24 to create a best-in-class PCV that provides broader coverage and better immune responses than standard-of-care vaccines."
"These data support that objective and demonstrate the potential of our PCV franchise, including VAX-31, our 31-valent PCV candidate."
In the Phase 2 study in adults aged 65 and older, VAX-24 demonstrated robust OPA immune responses for all 24 serotypes at all doses studied, confirming the prior adult study results.
The VAX-24 2.2mcg dose, which Vaxcyte plans to advance to Phase 3, showed an overall improvement in immune responses vs. PCV20 relative to the results from the prior Phase 2 study in adults aged 50-64.
And the six-month safety data from both studies showed safety and tolerability results for VAX-24 similar to PCV20 at all doses studied.
The unedited announcement is posted at this link.
Other pneumococcal (PCV13, PCV15, PCV20, PCV24) vaccine news is posted at Precision Vaccinations.

Gilead Sciences, Inc. today announced positive results from several COVID-19 clinical and real-world evidence studies being presented at the 33rd European Congress of Clinical Microbiology & Infectious Diseases.
For example, a Phase 3 clinical study demonstrated that Veklury® (remdesivir) was generally well tolerated in people with moderate to severe renal impairment.
Additional data includes a retrospective real-world study that demonstrated that Veklury treatment is associated with a lower risk of death from COVID-19 for people with cancer.
A separate real-world analysis demonstrated that Veklury is also associated with reduced hospital readmission risk in immunocompromised patients hospitalized with COVID-19.
"The breadth of clinical and real-world evidence data presented at ECCMID further support the strong efficacy and safety profile of Veklury," said Frank Duff, MD, Senior Vice President, Virology Therapeutic Area Head, Gilead Sciences, in a press release on April 16, 2023.
"Since the beginning of the (COVID-19) pandemic, Veklury has played a critical role in the treatment of hospitalized patients with COVID-19."
"The real-world data further demonstrates its role in reducing mortality and hospital readmission rates in vulnerable patient populations, including people living with cancer and other immunosuppressed conditions."
Additionally, results from a Phase 1 clinical study evaluating the safety, tolerability, and pharmacokinetics of obeldesivir, previously known as GS-5245, a novel investigational oral compound developed by Gilead for the treatment of SARS-CoV-2 infection, showed obeldesivir reaches expected therapeutic plasma concentrations for the treatment of COVID-19.
Moderna, Inc. and Merck today announced the first presentation of detailed results from the Phase 2b clinical trial evaluating mRNA-4157 (V940).
In the overall intention-to-treat population, adjuvant treatment with mRNA-4157 in combination with KEYTRUDA demonstrated a statistically significant and clinically meaningful improvement in recurrence-free survival (RFS) and reduced the risk of recurrence or death by 44% (HR=0.56 [95% CI, 0.309-1.017]; one-sided p value=0.0266) compared with KEYTRUDA alone.
Dr. Kyle Holen, M.D. Moderna's Senior Vice President and Head of Development, Therapeutics, and Oncology commented in a press release on April 16, 2023, "The profound observed reduction in the risk of recurrence-free survival suggests this combination may be a novel means of potentially extending the lives of patients with high-risk melanoma."
"We look forward to starting the Phase 3 melanoma trial soon and expanding testing to lung cancer and beyond."
mRNA-4157 is a novel investigational mRNA-based individualized neoantigen therapy consisting of a single synthetic mRNA coding for up to 34 neoantigens that is designed and produced based on the unique mutational signature of the DNA sequence of the patient's tumor.
Individualized neoantigen therapies prime the immune system so patients can generate a tailored antitumor response specific to their tumor mutation signature.
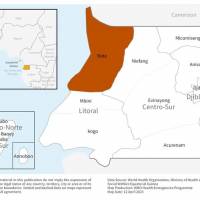
The Global Polio Eradication Initiative recently announced two African countries reported new polio cases involving vaccine-derived types.
As of April 11, 2023, the Democratic Republic of the Congo reported six circulating vaccine-derived poliovirus type 2 (cVDPV2) cases from the Kasai Oriental, Haut Katanga, and Tshopo provinces. During 2023, there have been 14 cases.
And in Benin, a second cVDPV2 case was confirmed in 2023.
Additionally, Burundi reported three cVDPV2-positive environmental samples, and Somalia confirmed one sample last week.
Previously, the World Health Organization (WHO) confirmed the spread of poliovirus remained a Public Health Emergency of International Concern.
Therefore, the WHO's International Travel and Health recommends travelers to polio-affected areas be fully vaccinated against polio.
Various polio vaccines are listed at Vax-Before-Travel.
A meeting of the U.S. FDA's Peripheral and Central Nervous System Drugs Advisory Committee is scheduled for June 9, 2023.
This FDA Committee's digital presentation will discuss the supplemental biologics license application for LEQEMBI™ (lecanemab) solution for intravenous infusion, submitted by Eisai, Inc., for treating early Alzheimer's disease (AD).
The Committee will discuss the confirmatory study, BAN2401-G000-301, conducted to fulfill post-marketing requirement 4384-1, detailed on January 6, 2023, FDA approval letter.
Confirmatory studies verify and describe a product's clinical benefit after receiving an FDA accelerated approval. Accordingly, its application was granted Priority Review, with a Prescription Drug User Fee Act action date of July 6, 2023.
LEQEMBI is a humanized immunoglobulin gamma 1 monoclonal antibody, not a preventive vaccine.
As of April 15, 2023, the FDA has not approved any Alzheimer's vaccine candidate.
FDA advisory committees provide independent expert advice on topics or specific products to help the agency make sound decisions based on the available science. Advisory committees make non-binding recommendations.
The FDA generally follows these recommendations but is not legally bound to do so.