Search API
While malaria vaccines have become more available in Africa during 2023, they are not available in the Region of the Americas, where they are needed to protect children.
As disease-carrying mosquitos expand their range, tourists in vacation hot-spots such as the Republic of Costa Rica's breaches are left unprotected against this disease.
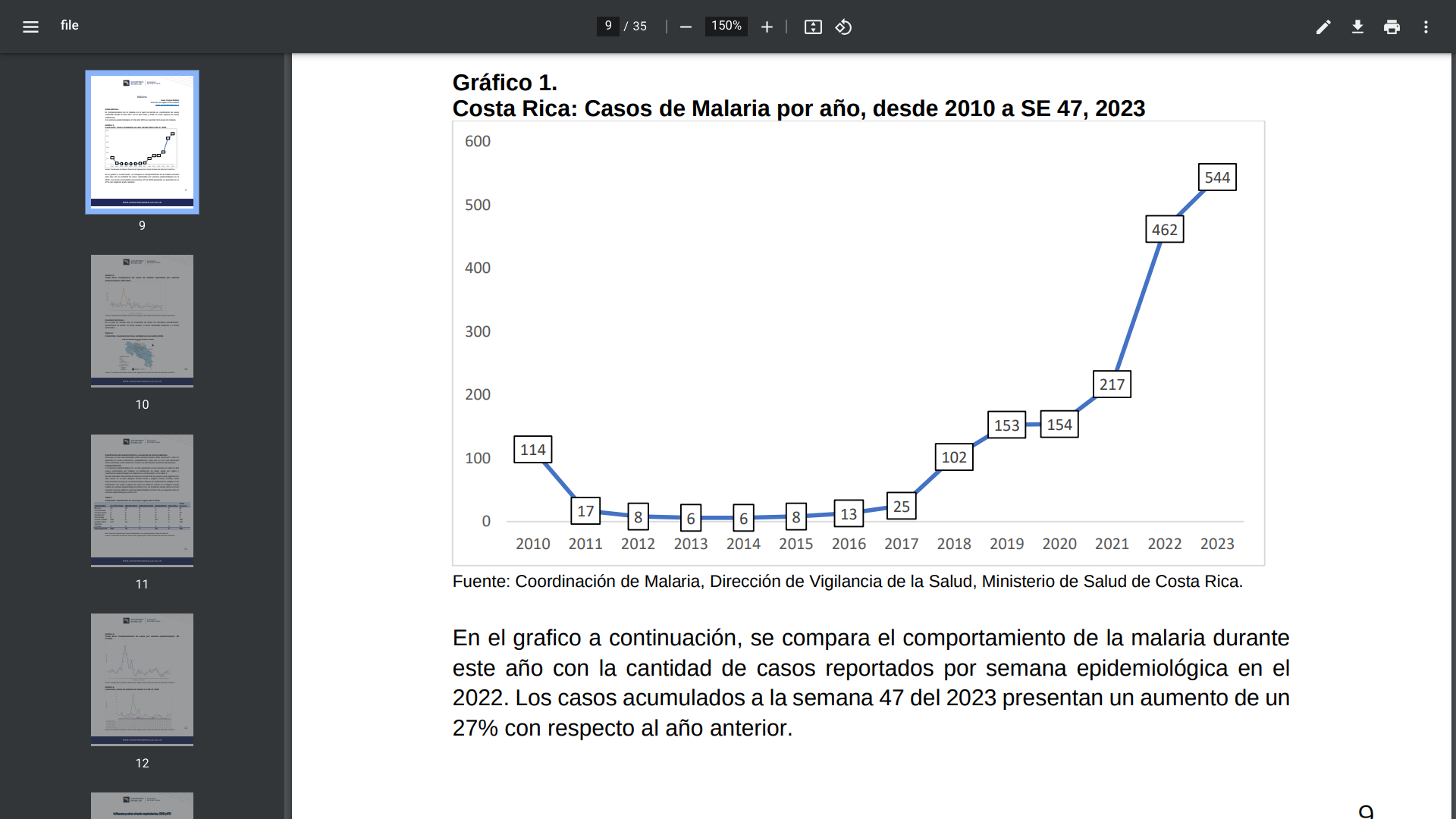
The Central American country of Costa Rica confirmed in December 2023 that there have been 544 malaria cases this year.
In 2022, Costa Rica's Health Ministry reported 406 locally-acquired malaria cases.
Globally, there were an estimated 249 million malaria cases and 426,000 deaths in 2022.
This year, the U.S. CDC recommends travelers visiting Costa Rica take prescription medicine to prevent malaria, but it can't suggest vaccinations yet.
As of October 2023, the World Health Organization (WHO) recommends two malaria vaccines for children: RTS,S/AS01, and R21/Matrix-M™.
The WHO expects these vaccines to have a positive public health impact.
And on December 21, 2023, WHO announced that it prequalified the R21/Matrix-M malaria vaccine. Prequalification status enables United Nations agencies to procure the vaccine for eligible countries.
Since most malaria cases in the United States are travel-related, the CDC may not expedite its approval for these new vaccines.
However, during 2023, local malaria cases have been confirmed in Florida, Maryland, and Texas.
Children located in a few African countries now have access to a second malaria vaccine, the R21/Matrix-M™ malaria vaccine.
As of December 21, 2023, the World Health Organization (WHO) has added this malaria vaccine to its list of prequalified vaccines, which increases access to vaccines procured by UNICEF and funding support for deployment by Gavi, the Vaccine Alliance.
In October 2023, WHO recommended that Oxford University's developed and Serum Institute of India manufactured R21/Matrix-M to prevent malaria in children following the advice of the WHO Strategic Advisory Group of Experts on Immunization and the Malaria Policy Advisory Group.
R21/Matrix-M integrates Novavax Inc.'s Matrix-M djuvant.
The initial malaria vaccine, Mosquirix™ RTS, S/AS01, obtained WHO prequalification status in July 2022.
"Today marks a huge stride in global health as we welcome the prequalification of R21/Matrix-M, the second malaria vaccine recommended for children in malaria-endemic areas, said Dr. Kate O'Brien, Director of WHO's Department of Immunization, Vaccines and Biologicals, in a press release on December 21, 2023.
"This is another step toward ensuring a healthier, more resilient future for those who have lived for too long in fear of what malaria could do to their children. Together with our partners, we are united in pursuing a malaria-free future, where every life is shielded from the threat of this disease."
Malaria is a mosquito-borne disease causing a high burden on children in the African Region, where nearly half a million children die from the disease each year. Globally, in 2022, there were an estimated 249 million malaria cases and 608,000 malaria deaths across 85 countries.
In the United States, about 2,000 malaria cases are detected in international travelers annually. However, local malaria cases in the U.S. (Arkansas, Florida, Maryland, Texas) were detected in 2023.
These WHO-prequalified malaria vaccines are not available in the U.S.
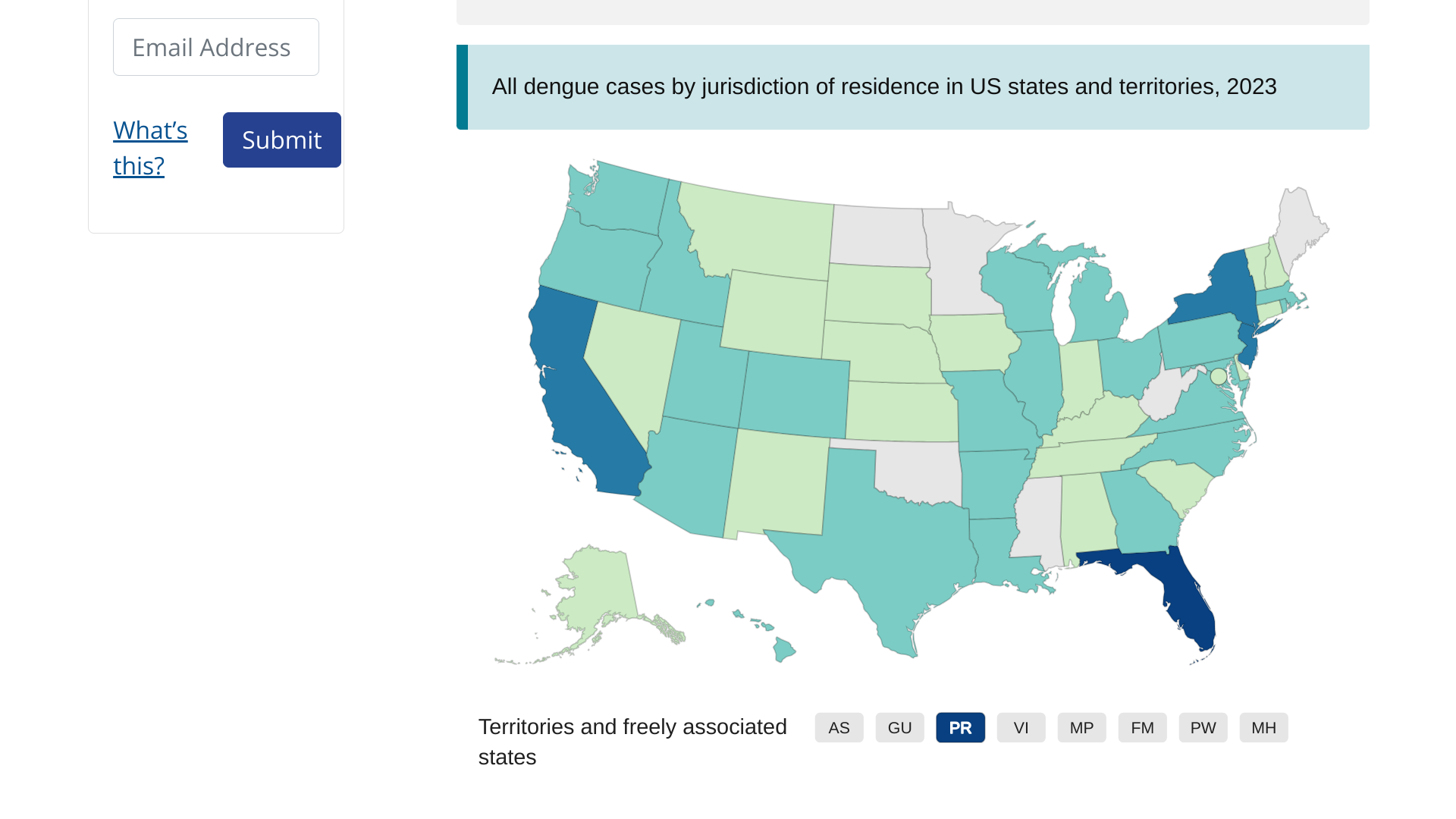
According to several health agencies, the global risk assessment related to dengue virus outbreaks increased in late December 2023.
The European Centre for Disease Prevention and Control reported that as of November 2023, over 4.5 million cases and over 4,000 dengue-related deaths have been reported from 80 countries/territories globally.
For example, the U.S. CDC reissued Level 1 - Practice Usual Precautions, Travel Health Notices on December 14, 2023, for countries located in Africa, Asia, the Americas, the Middle East, and the Pacific Islands.
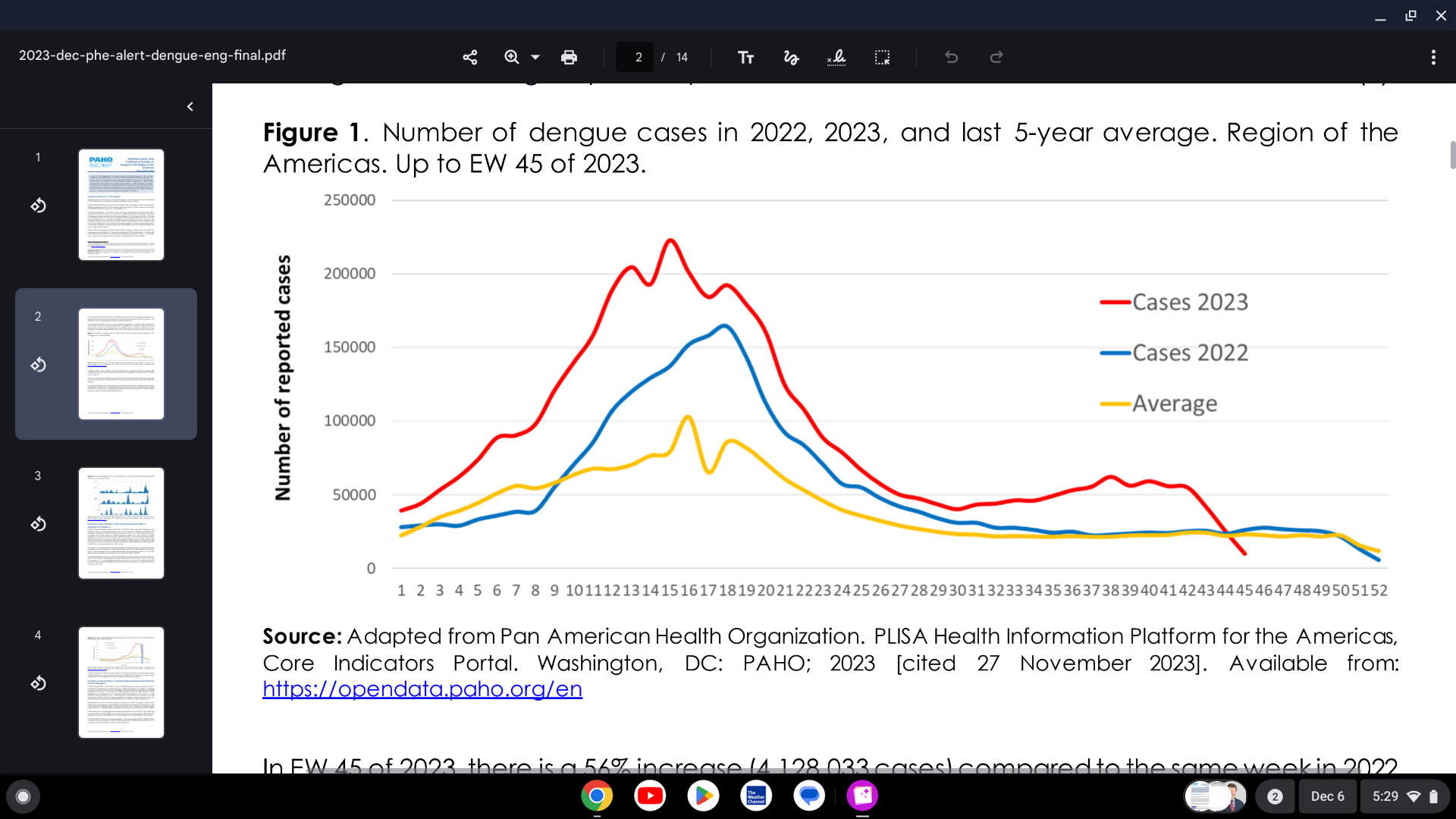
In the Americas, the Pan American Health Organization (PAHO) confirmed that 2023 is the year with the highest historical record of dengue cases, registering more than 4.1 million new infections, exceeding 2019, when about 3.1 million cases were reported.
Several factors are associated with the increasing risk of dengue epidemics, such as the changing range in elevation of Aedes aegypti mosquitoes, especially in previously dengue native areas, stated the PAHO on December 12, 2023.
Because mosquito bites spread dengue, all travelers to these areas, including southeast Florida and Puerto Rico, are at risk.
Since dengue can become severe within a few hours, usually requiring hospitalization, prevention options are essential.
In addition to avoiding mosquito bites, dengue is a vaccine-preventable disease.
As of late 2023, two approved dengue vaccines and several vaccine candidates are in development. These vaccines have limited availability, based on which country you are located in.

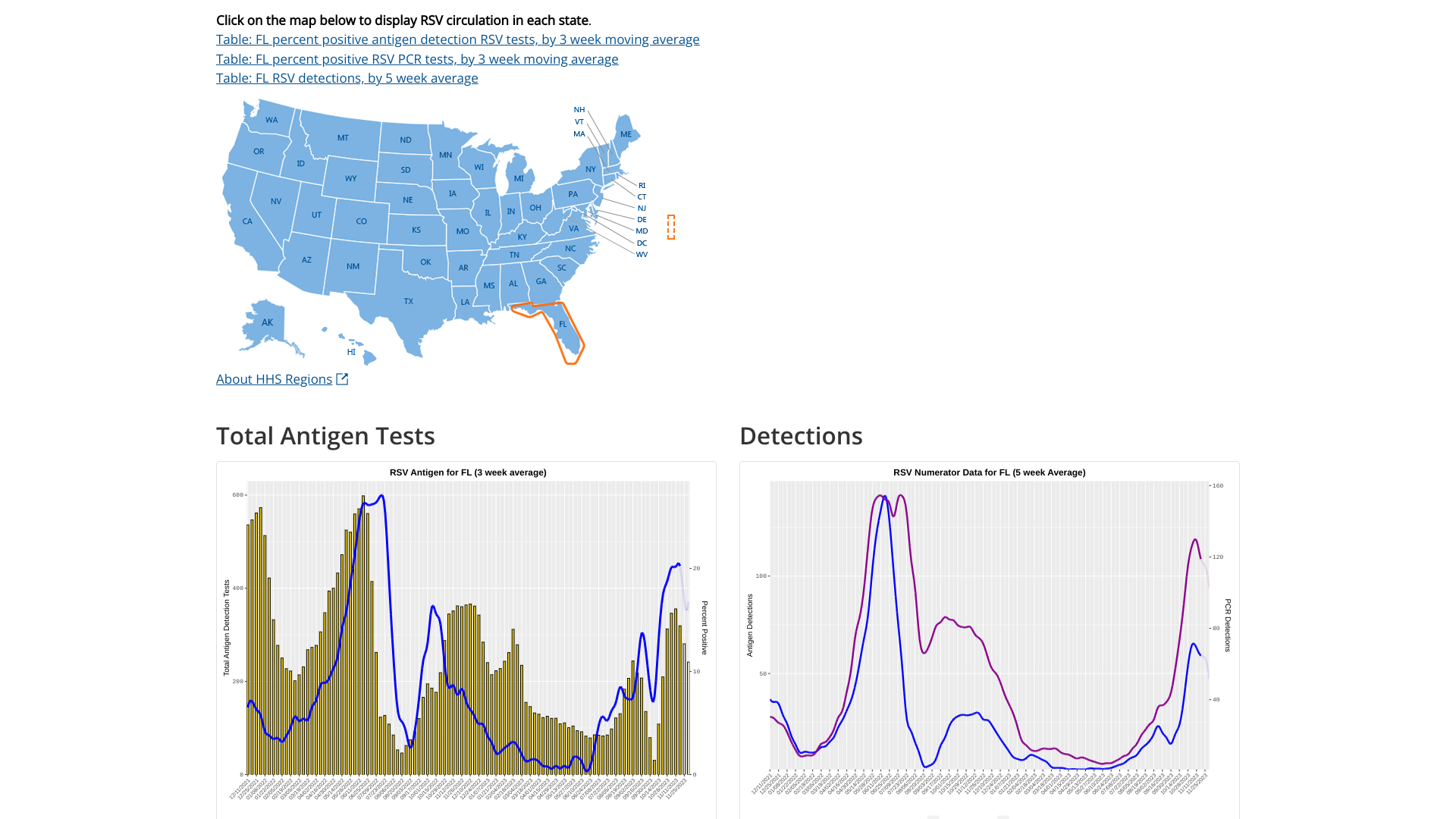
Since most respiratory syncytial virus (RSV) activity in the United States is initially recorded in the state of Florida, watching for the bending of the curve offers insights regarding future cases.
As of December 8, 2023, the U.S. Centers for Disease Control and Prevention (CDC) updated its RSV detection graphs to display the 5-week moving average. RSV infections typically occur during late fall, winter, and early spring.
There are variations in the timing of RSV outbreaks between regions and communities in the same region.
For Florida, the two charts indicate RSV's peak was in late November 2023.
Separately, the Florida Department of Health reported that as of December 2, 2023, RSV activity was decreasing in hospital admissions, positivity tests, and ER visits.
But, there was an RSV outbreak in Pinellas County.
From a prevention perspective, both RSV vaccines are available at most clinics and pharmacies in Florida. However, the CDC recently confirmed the percentage of seniors receiving an RSV vaccine was just 15.9%.
Unfortunately, the RSV antibody passive immunization for infants, Beyfortus™, remains in limited supply.
Virtually all children get an RSV infection by the time they are two years old. Most of the time, RSV will cause a mild, cold-like illness. RSV antibodies can help protect children from severe disease from an RSV infection.
RSV can be dangerous for infants and young children. Each year, thousands are hospitalized due to RSV infection, says the CDC.
The Pan American Health Organization (PAHO) issued an Epidemiologic Alert confirming a significant increase in dengue during the second half of 2023 in several countries of the Region of the Americas, especially in Central America and the Caribbean.
As of December 5, 2023, the Americas set a new record number of dengue cases with over 4.1 million patients.
According to the seasonal pattern of dengue and the current rainy season, the PAHO called for the intensification of preparedness actions within healthcare services to facilitate access and proper management of patients ahead of the Southern Hemisphere's peak season.
In 2023, through week #45, 1,954 dengue-related deaths were reported in the Region of the Americas (case fatality rate [CFR]: 0.048%).
The most significant number of dengue cases were reported in Brazil, with 2,909,404 cases.
Of the 6,340 severe dengue cases reported in 2023, the highest numbers were recorded in Brazil with 1,474 cases, Colombia with 1,390, Mexico with 1,142, Peru with 1,065, and Bolivia with 640 cases.
In addition, a notable increase in the notification of locally transmitted cases has been observed in places such as The Bahamas and the state of Florida.
Given this situation, the PAHO / World Health Organization urges Member States to implement appropriate actions at the level of patient care services, including triage, diagnosis, and timely and proper treatment of dengue cases and other arboviruses such as Zika.
As of December 2, 2023, the PAHO indicated 31,780 Zika cases across the Americas this year.
From a protection perspective, two dengue vaccines have been authorized in various countries since October 2022.
As the global dengue virus outbreak continues in about 80 countries with over 4.5 million dengue cases and over 4,000 dengue-related deaths, new data from Florida indicates there may be fewer cases this year.
Florida Health's Mosquito-Borne Disease Surveillance Report #46 confirmed 458 travel-associated dengue cases and 142 locally acquired dengue cases in 2023.
As of November 18, 2023, most of the travel-related dengue cases have been associated with visitors from Cuba (272).
And Miami-Dade Country has confirmed the most local dengue cases (133).
In 2022, Florida reported 903 travel-associated and 68 locally-acquired dengue cases.
From a prevention perspective, the U.S. FDA-approved Dengvaxia® vaccine is available but is seldom administered because of required diagnostic testing.
Dengvaxia (CYD-TDV) is a live attenuated tetravalent chimeric vaccine made using recombinant DNA technology.
Recently, the FDA extended Dengvaxia's approval to include children aged 9–16 years with laboratory-confirmed previous dengue virus infection and living in areas where dengue is endemic.
However, the vaccine is not approved for use in U.S. travelers who are visiting but not living in an area where dengue is common.
Endemic areas can include some U.S. territories and freely associated states.
A new dengue vaccine, QDENGA®, has recently been approved in various countries, without a pre-administration test requirement.
QDENGA (TAK-003) prevents dengue fever and/or severe dengue caused by any of the four serotypes.
The World Health Organization (WHO) today announced that 331,200 doses of Mosquirix™ RTS, S/AS01, arrived in the Republic of Cameroon.
Mosquirix is a recombinant malaria vaccine with the P. falciparum circumsporozoite protein.
The delivery on November 21, 2023, is the first to a country located on the Gulf of Guinea not previously involved in the WHO malaria vaccine pilot program and signals that scale-up of vaccination against malaria across the highest-risk areas on the African continent will begin shortly.
A further 1.7 million doses of Mosquirix are expected to arrive in Burkina Faso, Liberia, Niger, and Sierra Leone in the coming weeks, with additional African countries set to receive doses in the months ahead.
Since 2019, Ghana, Kenya, and Malawi have been administering the vaccine in a schedule of 4 Mosquirix doses from around five months of age in selected districts as part of the pilot program known as the Malaria Vaccine Implementation Programme (MVIP).
More than 2 million children have been reached with the malaria vaccine in three African countries through MVIP – resulting in a 13% drop in all-cause mortality in children age eligible to receive the vaccine and substantial reductions in severe malaria illness and hospitalizations.
Nearly every minute, a child under five dies of malaria in Africa.
In 2021, there were 247 million malaria cases globally, which led to 619,000 deaths in about 84 countries.
Of these deaths, 77% were children under five years of age.
Approximately 95% of global malaria cases and 96% of related deaths in 2021.
The U.S. Centers for Disease Control and Prevention (CDC) has issued various alerts for malaria-endemic countries, including Costa Rica.
The CDC has recently confirmed autochthonous (local) malaria cases in Florida (seven), Texas, Maryland, and Arkansas.
As of November 22, 2023, malaria vaccines are unavailable in the U.S.
In a Lancet Respiratory Medicine news article published on November 6, 2023, Sean O'Leary, MD, chair of the American Academy of Pediatrics (AAP)'s Committee on Infectious Diseases, stated the nationwide shortage of Beyfortus™ (Nirsevimab-alip), a newly approved respiratory syncytial virus (RSV) monoclonal antibody, could have been predicted.
"I would've predicted pretty high demand. I think probably too much was made of vaccine hesitancy and refusal..." wrote Dr. OLeary.
Sanofi, the producer of Beyfortus, stated on October 26, 2023, 'Despite an aggressive supply plan built to outperform past pediatric immunization launches, demand for this product, especially for the 100 mg doses used primarily for babies born before the RSV season, has been higher than anticipated.'
Sanofi collaborates closely with the U.S. Centers for Disease Control and Prevention (CDC) to ensure equitable distribution of available doses through the Vaccines for Children Program.
The CDC recently issued an advisory with recommendations for clinicians to prioritize 100-milligram doses for infants younger than six months and those with underlying medical conditions that predispose them to severe RSV.
Beyfortus is the second monoclonal antibody developed to prevent RSV in young children.
The AAP has recommended Arexis AB's palivizumab (Synagis) for high-risk infants and young children during an active RSV season.
Synagis was approved for initial use in the U.S. by the FDA in 1998. It is not an RSV vaccine but can help passively protect children with monthly dosing.
As of November 7, 2023, the RSV season began in Florida and has spread throughout the United States, impacting certain areas.

Moffitt Cancer Center today announced researchers are working to improve the efficacy of neoantigen-targeted cancer vaccines by better understanding whether primary or metastatic tumors should be used to produce the personalized vaccine.
On November 5, 2023, these cancer specialists launched a study evaluating primary and metastatic tumor pairs from 45 patients with several solid tumor types, including melanoma, bladder, head and neck cancers, and non-small cell lung cancer.
Results presented at the Society for Immunotherapy of Cancer annual meeting show that melanoma, bladder, and head and neck tumors share a high percentage of mutations between primary and metastatic tumors.
However, other solid tumors, such as esophageal and non-small cell lung cancer, share less.
Whole exome sequencing was used to identify somatic alterations, which are genetic mutations or DNA alterations that may impact the type of antigens produced by the cancer cells that the vaccine can then target.
Dr. Ahmad Tarhini, Director, Cutaneous Clinical and Translational Research at Moffitt, commented in a press release, "Our analysis demonstrates genetic variations that exist when comparing paired primary and metastatic tumors that appear to vary by histology."
"Variants are potentially undergoing negative selection supported by the preferential loss of out-of-frame events in metastatic tumors."
Understanding the clonal structure will be vital to predicting neoantigens for effective neoantigen-based vaccines, where oncogenic drivers can be prioritized and used to determine the primary clones.
Tarhini and the Moffitt team continue this work, expanding their study to include paired tumor samples from 600 additional patients.
As Florida's only National Cancer Institute-designated comprehensive cancer center and one of only 30 leading cancer centers in the U.S. participating in the National Comprehensive Cancer Network, Moffitt is at the forefront of cancer centers worldwide.

