Search API
Before 2023, there were no immunizing tools to protect older adults from illness and death due to respiratory syncytial virus (RSV). This year, the U.S. Food and Drug Administration approved two RSV vaccines for older adults.
According to an Original Article published today by the New England Journal of Medicine, an mRNA-based vaccine was found to be very effective at protecting healthy adults.
In this ModernaTX, Inc. sponsored ongoing, randomized, double-blind, placebo-controlled, phase 2/3 clinical trial, participants were randomly assigned, in a 1:1 ratio, adults 60 years of age or older to receive one dose of mRNA-1345 (50 μg) or placebo.
The two primary efficacy endpoints were preventing RSV-associated lower respiratory tract disease with at least two signs or symptoms and at least three signs or symptoms. A key secondary efficacy endpoint was the prevention of RSV-associated acute respiratory disease. Safety was also assessed.
Overall, 17,793 participants were assigned to receive the mRNA-1345 vaccine candidate. The median follow-up was 112 days (range, 1 to 379).
The primary analyses were conducted when at least 50% of the anticipated cases of RSV-associated lower respiratory tract disease had occurred.
Vaccine efficacy was 83.7% (95.88% confidence interval [CI], 66.0 to 92.2) against RSV-associated lower respiratory tract disease with at least two signs or symptoms and 82.4% (96.36% CI, 34.8 to 95.3) against the disease with at least three signs or symptoms. Vaccine efficacy was 68.4% (95% CI, 50.9 to 79.7) against RSV-associated acute respiratory disease.
Protection was observed against both RSV subtypes (A and B) and was generally consistent across subgroups defined according to age and coexisting conditions.
Participants in the mRNA-1345 group had a higher incidence than those in the placebo group of solicited local adverse reactions (58.7% vs. 16.2%) and systemic adverse reactions (47.7% vs. 32.9%). Most of these reactions were mild to moderate in severity and were transient.
However. serious adverse events occurred in 2.8% of the participants in each trial group.
In summary, these researchers wrote, 'This phase 2–3 efficacy trial showed that a single 50-μg dose of the mRNA-1345 vaccine in adults 60 or older was efficacious against a spectrum of RSV-confirmed respiratory disease.'

A Yale University-led study published today in the journal Pediatrics announced the rate of invasive pneumococcal disease (IPD) in children decreased by about 72% (incidence rate ratios 0.28, 95% CI 0.18–0.45) over twenty years.
This research found IPD rates continued to decline after the replacement of PCV7 with PCV13.
And during the recent pandemic, the rate of IPD among children aged <18 years reached 1.6 per 100,000, the lowest incidence observed over the 20 years.
In the PCV13 vaccine era, approximately one-third of the IPD cases in children aged >5 years had at least one underlying condition.
Serotypes 19A and 7F contributed 342 (48.9%) of all cases before implementation of PCV13 (2002–2010).
Serotype 3 (31, 8.6%), and non-PCV13 serotypes 15B/C (39, 10.8%), 33F (29, 8.0%), 23B (21, 0.8%), and 35B (17, 4.7%) were responsible for 37.8% of cases in PCV13 era (2011–2021).
Furthermore, penicillin nonsusceptibility declined (9.8% vs. 5.3% in the pre-/late PCV13 era, P = .003). However, it has become more common among non-PCV13 serotypes than vaccine serotypes (14.8% vs 1.4%, P < .001).
These Yale Child Health Research Center researchers concluded that robust ongoing surveillance networks are critical for identifying emerging pneumococcal serotypes and developing next-generation vaccine formulations.
As of December 2023, various pneumococcal vaccines are available at clinics and pharmacies in Asia, Europe, Japan, the U.S., and the United Kingdom.

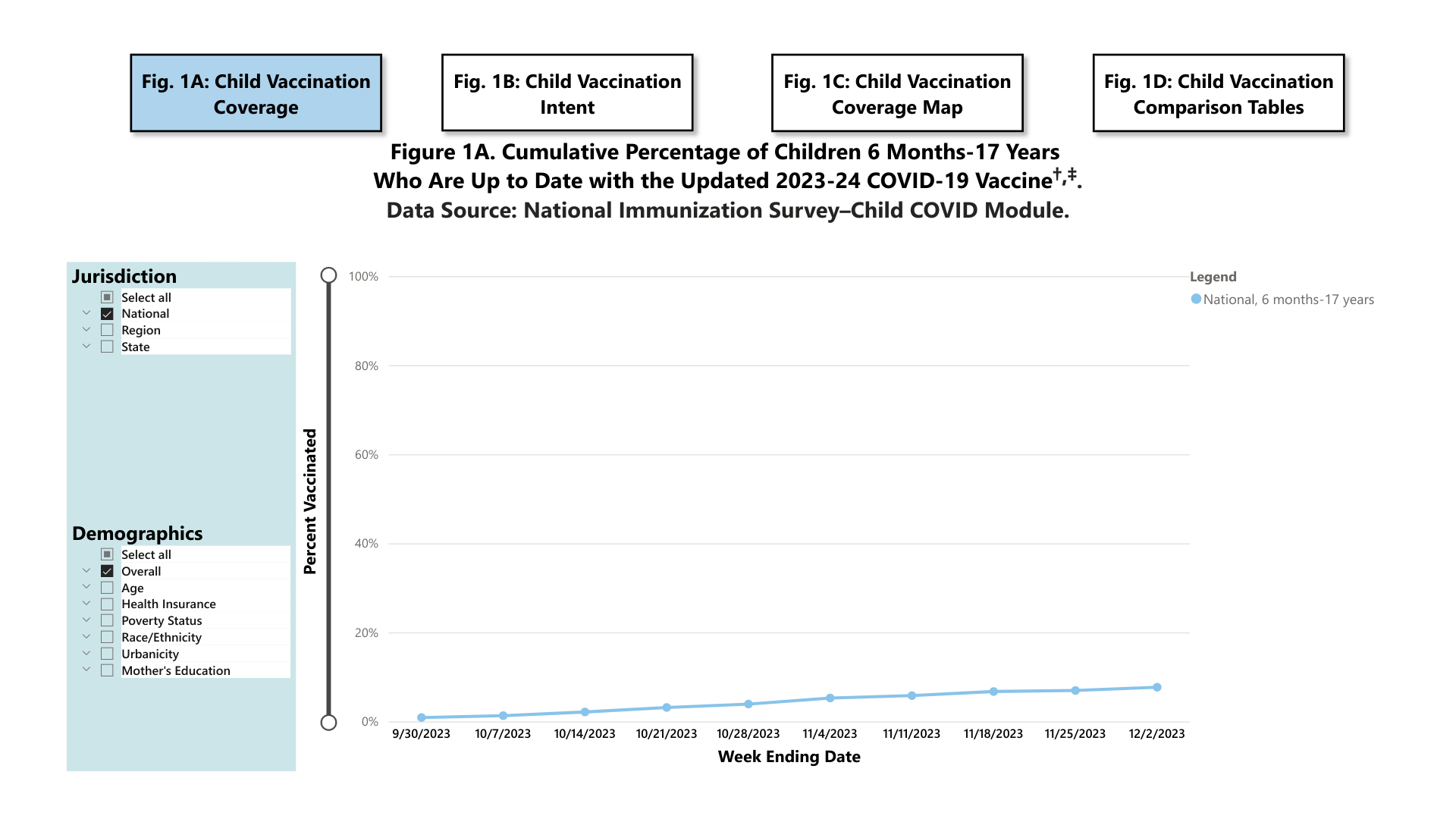
The U.S. CDC's COVIDVaxView COVID-19 Vaccination Dashboard was updated today, reflecting current trends in children's, adults, and pregnant women's vaccination rates.
As of December 13, 2023, the CDC confirmed:
As of December 2, 2023, 7.7% (95% Confidence Interval: 6.5%-8.9%) of children (six months to 17 years) were reported to be up to date with the 2023-24 COVID-19 vaccine. An additional 18.6% (15.9%-21.3%) of children had a parent who said they planned to get their child vaccinated.
And 17.2% (95% Confidence Interval: 16.3%-18.1%) of adults reported receiving an updated 2023-24 COVID-19 vaccine since September 14, 2023. An additional 14.6% (13.4%-15.9%) said they plan to get vaccinated.
Furthermore, 9.6% of pregnant women had received the updated 2023-24 COVID-19 vaccine. Vaccination coverage was highest among non-Hispanic Asian (16.5%) pregnant women and lowest among non-Hispanic Black (3%) women.
Additional COVID-19 vaccination data by demographic characteristics at the national level and overall estimates by jurisdiction are available at this CDC link.
Clover Biopharmaceuticals, Ltd. today announced that enrollment of the first participants has been completed in a Phase 1 first-in-human clinical study evaluating the company's RSV PreF-Trimer subunit vaccine candidate, SCB-1019.
"We are pleased to be the first vaccine company based in China developing an RSV prefusion-stabilized F (PreF) vaccine to enter the clinical trial stage, establishing our leadership position in the space, which demonstrates the value of our validated Trimer-Tag platform and capabilities of our R&D team," said Joshua Liang, Chief Executive Officer and Board Director of Clover, in a press release on December 12, 2023.
"RSV vaccines remain a high unmet medical need, especially in China where no domestic RSV PreF vaccines have entered the clinical stage to date, but also globally where there is an opportunity for differentiation."
SCB-1019 is a bivalent RSV-A/RSV-B vaccine candidate based on the prefusion-stabilized F (PreF) protein leveraging the validated Trimer-Tag platform and proprietary stabilizing PreF mutations.
The new clinical trial is a randomized, placebo-controlled study to assess the safety, reactogenicity, and immunogenicity of SCB-1019 in young and older adults at multiple dose levels and in different formulations. Safety and immunogenicity results are expected by the second half of 2024.
As of December 13, 2023, there are approved RSV vaccines in various countries, and several vaccine candidates are conducting clinical trials.

Eisai Co., Ltd. and Biogen Inc. today announced that humanized anti-soluble aggregated amyloid-beta (Aβ) monoclonal antibody LEQEMBI® Intravenous Infusion (200 mg, 500mg, lecanemab) is scheduled to launch in Japan on December 20, 2023.
LEQEMBI received manufacturing and marketing approval for the indication of slowing progression of mild cognitive impairment and mild dementia due to Alzheimer's disease (AD) in Japan on September 25, 2023.
The pending launch in Japan marks the second country to have LEQEMBI on the market, following the U.S. Food and Drug Administration (FDA) approval in July 2023.
In the U.S., treatment with LEQEMBI should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was initiated in clinical trials, says the FDA.
"The availability of LEQEMBI opens a new era in the treatment of AD, potentially giving patients and their families additional valuable time together and further positions Japan as a leader in caring for an elderly population," said Christopher A. Viehbacher, President and Chief Executive Officer of Biogen, in a press release on December 13, 2023.
"We will work alongside Eisai to engage the medical community and support the patient journey, especially early diagnosis, as mounting evidence suggests early intervention may provide a greater impact on disease progression."

More than 20 anthrax-related deaths have been reported in five countries in Africa since the start of 2023.
As of December 11, 2023, a total of 1,166 suspected and 37 confirmed cases have been recorded in Kenya, Malawi, Uganda, Zambia, and Zimbabwe, according to data reported to the World Health Organization (WHO).
Of the five countries, Zambia is witnessing its largest anthrax outbreak since 2011, with 25 confirmed cases and four deaths. Only sporadic cases have previously been reported in animals and humans in the country.
Over 400,000 vaccine doses have been earmarked for high-risk districts in Zambia's western province.
Annually, human anthrax infections are the highest in Africa, the Middle East, and Central and South Asia, says the WHO
"To end these outbreaks, we must break the infection cycle by first preventing animal disease. We are supporting the ongoing national outbreak control efforts by providing expertise as well as reinforcing collaboration with partner agencies for a common approach to safeguard human and animal health," said Dr Matshidiso Moeti, WHO Regional Director for Africa, in a press release.
Humans become infected with anthrax, a zoonotic disease, through contact with disease carrying animal carcasses or exposure to contaminated animal products. Rare person-to-person transmissions have been reported with cutaneous anthrax, which accounts for more than 95% of human cases worldwide.
Cutaneous anthrax usually develops 1–7 days after exposure, but incubation periods up to 17 days have been reported.
A study published by the journal Nature Biology estimated that 1.83 billion people (95% credible interval (CI): 0.59–4.16 billion) live within regions of anthrax risk, but most of that population faces little occupational exposure.
Individuals potentially exposed to anthrax spores may be provided with prophylactic treatment. Anthrax responds well to antibiotics, says the WHO.
In the United States, Emergent BioSolutions Inc.'s CYFENDUS™ (Anthrax Vaccine Adsorbed, Adjuvanted) was approved on July 20, 2023, as a two-dose anthrax vaccine for Post-Exposure Prophylaxis use.
However, the U.S. CDC says vaccination against anthrax is not recommended for travelers and is not available for civilian travelers.

GSK plc today announced the recipients of the inaugural grant program of the COiMMUNITY Initiative, a multipronged effort to support the design of a more systematic, collaborative and equitable approach to helping increase adult immunization rates in the United States.
Grant recipients include but are not limited to the American Lung Association, American Pharmacists Association (APhA), APhA Foundation, Asian Health Coalition, Global Health Living Foundation, Immunize Kansas Coalition, Pharmacy Quality Alliance, and The Arizona Partnership for Immunization.
Each grant-funded project receives between $50,000 and $175,000 out of a total of $1 million to help address long-standing barriers to adult immunization in the U.S.
Rob Truckenmiller, Senior Vice President, Head of U.S. Vaccines, GSK, commented in a press release on December 12, 2023, "Getting ahead of vaccine-preventable disease starts by investing in organizations who know their communities best, and I can't think of a more deserving group of recipients for our first-ever COiMMUNITY Initiative grants."
"With these grants, our goal is to support innovative ways to encourage adult vaccination and help mitigate health inequities at both the local and national levels."
The organizations that received funding submitted proposals that demonstrated innovative, ambitious, and actionable ideas for boosting adult vaccination, such as building vaccine confidence among underserved patient populations through trusted community partners, creating engaging multilingual educational materials, and developing resources to support pharmacists and local health departments.
Through the COiMMUNITY Initiative, GSK will continue to build on its commitment to help support long-lasting solutions to address adult immunization challenges through ongoing investments to empower stakeholders with actionable data, models, and best practices in adult vaccine confidence and delivery.