Search API
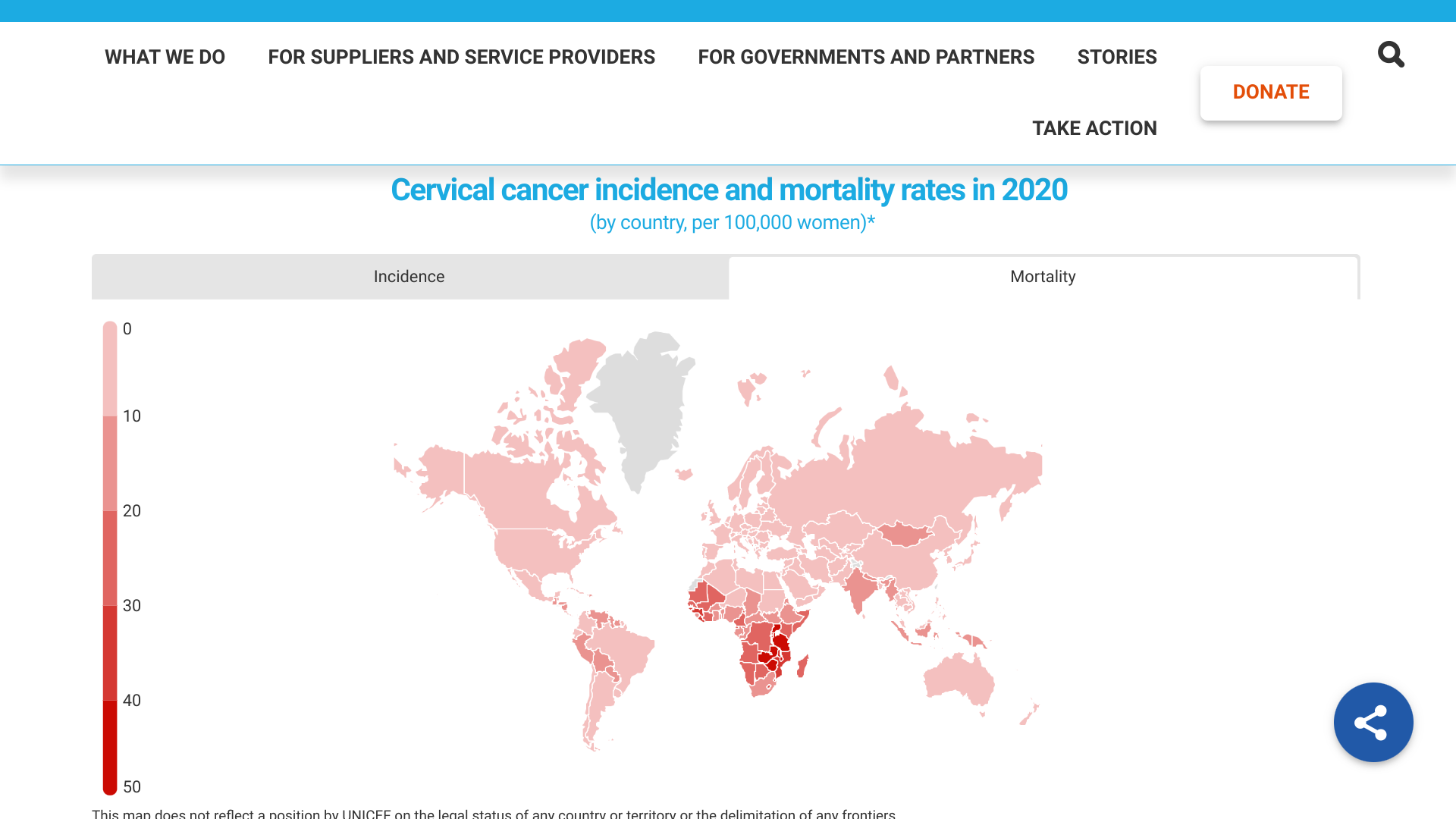
UNICEF recently announced it would supply 52 countries with human papillomavirus (HPV) vaccines. In 2023 alone, UNICEF will provide one in four countries worldwide with this life-saving vaccine.
And seven countries, Bangladesh, Cambodia, Eswatini, Kiribati, Mongolia, Nigeria, and Togo, intend to introduce HPV vaccines to their routine immunization programs in 2023.
On April 28, 2023, UNICEF confirmed just one in eight girls are vaccinated against HPV, the leading cause of cervical cancer.
And since 2019, HPV vaccination coverage has decreased by 15%, representing one of the largest backslides of any vaccine during the pandemic.
Oluwaseun Ayanniyi, a Contracts Specialist in the Vaccine Centre of UNICEF’s Supply Division in Copenhagen, commented that she is optimistic that significant ground can be regained to prevent cervical cancer.
HPV vaccination can help prevent certain cancers for boys and girls, says the U.S. CDC. These vaccines are generally available at health clinics and pharmacies in the U.S.
As of May 8, 2023, the U.S. Food and Drug Administration has approved various vaccines that can prevent certain sexually transmitted diseases such as Mpox.
CureVac N.V. today announced that the first participant was dosed in a combined Phase 1/2 study of multivalent, modified mRNA seasonal flu vaccine candidates developed in collaboration with GSK is being conducted in the U.S. and Belgium.
The tested multivalent vaccine candidates address all four WHO-recommended flu strains.
"Our clinically validated technology platform and second-generation mRNA backbone give us great confidence as we continue clinical development of novel vaccine candidates to address seasonal flu," said Dr. Myriam Mendila, Chief Development Officer of CureVac, in a press release on May 8, 2023.
"There are still unmet needs as seasonal flu is ever-evolving and immune responses to current vaccines remain a challenge, particularly in older adults."
"The flexibility, speed, and scalability of CureVac's end-to-end mRNA capabilities position us well to develop and deliver seasonal flu vaccines together with GSK that combat dominant strains of the season as they emerge."
As previously reported, in CureVac and GSK's ongoing Phase 1 trial in older and younger adults of a monovalent, modified mRNA seasonal flu vaccine candidate, preliminary data showed a favorable tolerability profile and no concerning safety signals. In addition, the preliminary immunogenicity data indicated strong hemagglutinin inhibition immune responses in line with a licensed flu comparator vaccine beginning at the lowest tested dose.
The CureVac-GSK infectious disease collaboration was first announced in July 2020.

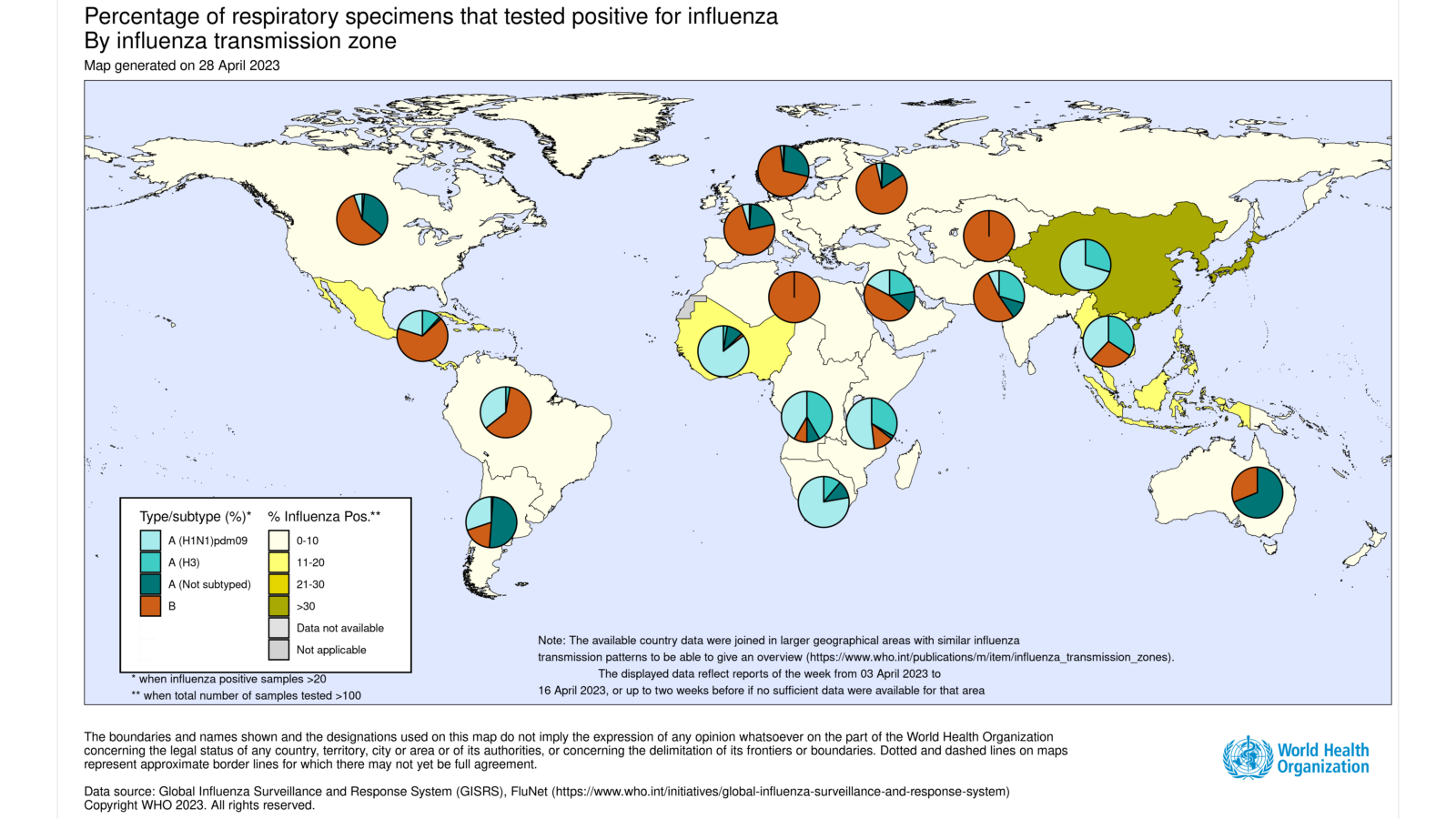
The World Health Organization (WHO) recently published Influenza Update N° 444, indicating that some Southern Hemisphere countries reported increased influenza detections.
On May 3, 2023, the WHO reported in the Caribbean and Central American countries, influenza activity of mainly influenza B/Victoria lineage viruses was low or below baseline in most countries.
Moreover, increases in influenza activity were reported in a few countries, and activity was moderate in Jamaica.
In tropical Africa, influenza detections were low in reporting countries. Influenza A virus detections outnumbered B virus detections.
In Southern Asia, influenza activity remained low, with influenza A(H3N2) predominant, followed by B/Victoria lineage viruses. However, increased activity was reported in Bhutan and Sri Lanka.
In South-East Asia, influenza activity remained elevated mainly due to detections in Malaysia and Singapore.
However, in Malaysia, activity decreased.
But there was an increased proportion of influenza A viruses over the past several weeks, and influenza A viruses predominated during this period.
On the other hand, influenza A(H3N2) viruses remained predominant in Singapore.
In the temperate zones of the southern hemisphere, influenza activity remained low.
And influenza activity increased slightly in Australia and Chile, and pneumonia surveillance in South Africa.
As of May 7, 2023, the WHO continues endorsing flu shots for the summer holiday season, wherever influenza outbreaks occur.
In the U.S., various influenza vaccines remain available at health clinics and community pharmacies.
As of early March 2023, about 173 million flu shots had been distributed during the 2022-2023 flu season.
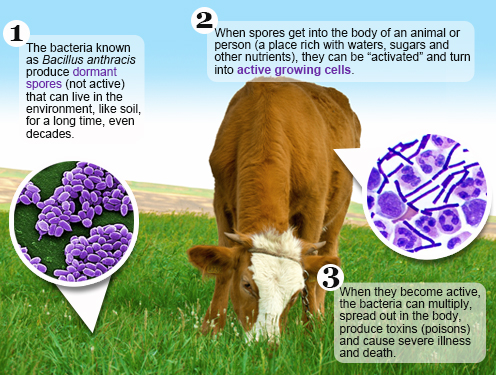
The Express News Service today reported 12 cases of anthrax, and one related fatality was recently reported in the villages of Dasmantpur.
Two others were being treated, and nine others are currently stable.
Preliminary examination suggests locals of Tentuliguda village reportedly consumed cow meat, after which they began showing symptoms of anthrax.
The Koraput district of Odisha, India, is an area endemic for anthrax.
Last year, anthrax was confirmed in 36 people in Tengwe in Zimbabwe, after consuming undercooked meat.
According to the U.S. CDC, cutaneous anthrax in humans is associated with exposure to infected animals or animal products and has a case fatality rate of up to 20% if untreated.
People can get sick with anthrax if they come in contact with infected animals or contaminated animal products. Anthrax can cause severe illness in both humans and animals.
The Anthrax Vaccine Adsorbed (AVA) protects against anthrax and does not contain any anthrax bacteria and cannot give people anthrax.
It is not typically available to the general public, says the CDC.
The Food and Drug Administration approved the vaccine for routine occupational use (before possible exposure) and post-event emergencies.
In the U.S., Emergent BioSolutions Inc.'s AV7909 (AVA) vaccine has been funded partly by the government for several years and is approaching authorization.
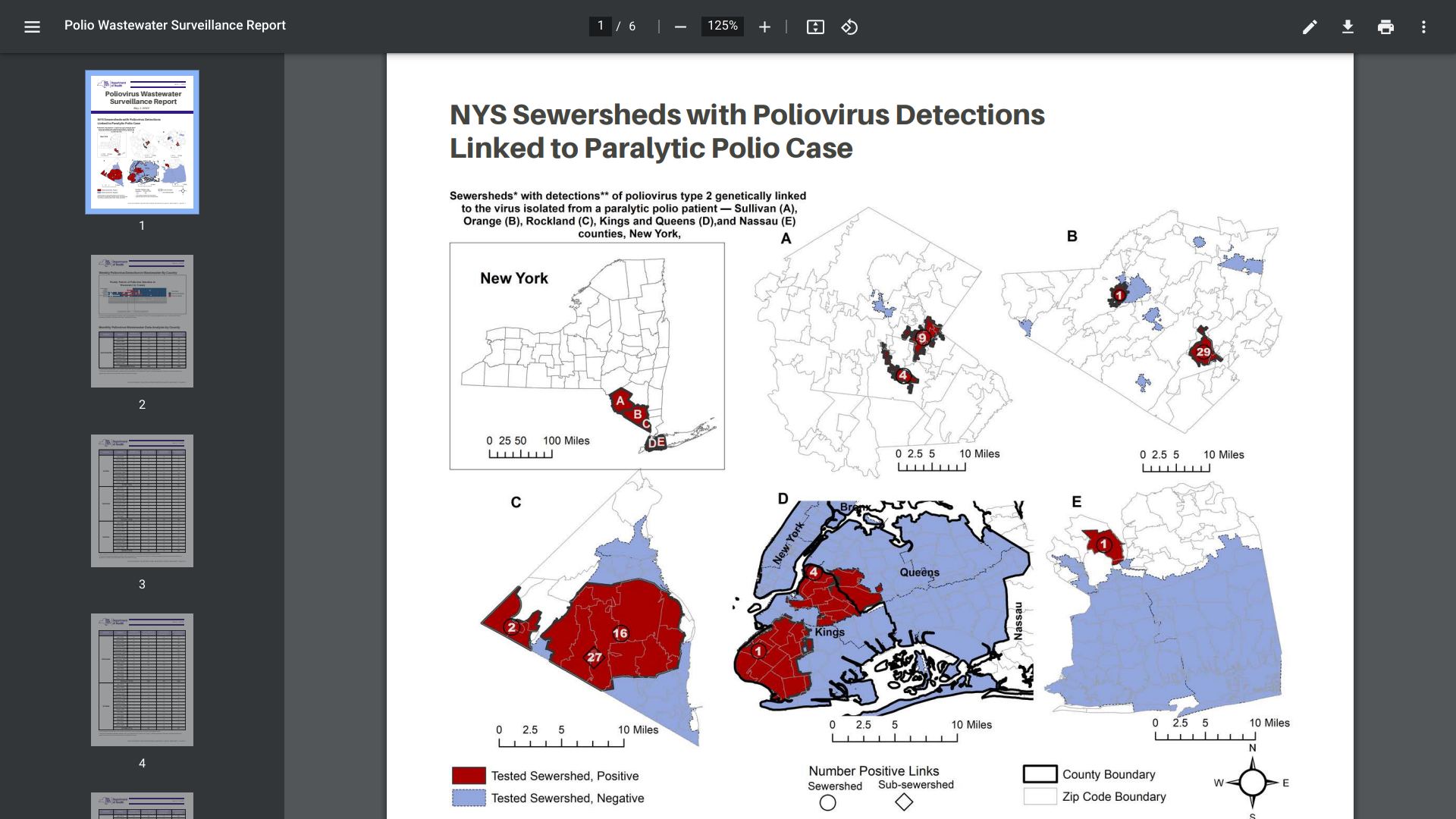
The New York (N.Y.) Department of Health recently issued an updated Poliovirus Wastewater Surveillance Report. Wastewater is sewage containing feces flushed down the toilet and other water from household drains.
Polio is highly contagious, and people can spread the virus even if they don't know they're sick. However, individuals infected with polio shed the virus in their stool, says N.Y.
On May 1, 2023, NY reported 1,170 samples had been tested for polio, with one positive sample of concern found in Rockland County.
In 2022, sequencing analysis confirmed the presence of poliovirus in a total of 100 positive samples of concern in Sullivan, Rockland, Orange, Nassau, and New York City.
N.Y.'s Health Department also clarified wastewater collected in sewer systems could not be a polio infection or transmission source for the general public. It does not contaminate drinking water, including tap water, streams, and lakes.
The U.S. CDC announced on November 30, 2022, that it would expand wastewater testing. Dr. José R. Romero, Director of CDC's National Center for Immunization and Respiratory Diseases, indicated that poliovirus testing would continue into early 2023.
In 2022, about 35 countries have confirmed similar findings from wastewater testing.
The U.S. CDC confirms polio is a vaccine-preventable disease.
And in 2023, N.Y. recommends a one-time polio vaccination booster for certain people. In addition, various polio vaccines are available at clinics and pharmacies in the U.S.
SIGA Technologies, Inc. today reported financial results ($8.3 million in revenue) for the three months ended March 31, 2023. Most of this revenue came from the Mpox oral treatment TPOXX®.
"First quarter product revenues primarily reflect the sale of oral TPOXX to the U.S. Department of Defense ("DoD"), which marks the third product delivery to the DoD within the past twelve months," said Phil Gomez, CEO of SIGA, in a press release on May 4, 2023.
"Including the $5 million of deliveries in the first quarter, we are targeting approximately $11 million of oral TPOXX deliveries to the DoD in 2023."
"In addition, based on the anticipated expiration this year of significant quantities of TPOXX held within the U.S. Government's Strategic National Stockpile ("SNS"), we are targeting for this year approximately $113 million of oral TPOXX deliveries to the SNS."
"We will continue to build and meet the demand for oral TPOXX across geographic regions worldwide and continue to work toward deliveries this year of IV TPOXX to the SNS."
TPOXX is a novel small-molecule antiviral known as tecovirimat and ST-246®, available as an orally administered and IV formulation for treating human disease caused by the variola virus.
TPOXX was approved in the U.S. (July 13, 2018), Canada, U.K., and Europe to treat smallpox and Mpox in 2022.
However, recent reports indicate uncertain product efficacy.
The U.S. CDC published Notes from the Field on April 28, 2023, describing New York City patients with Mpox who developed new lesions after completing tecovirimat treatment, suggesting post-treatment lesions might occur more commonly than previously reported.
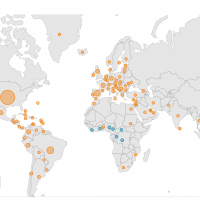
As of April 26, 2023, the CDC confirmed 87,078 Mpox cases and 130 related fatalities from 111 counties since May 2022.
And as of May 5, 2023, Bavarian Nordic JYNNEOS® (MVA-BN) vaccine remains available to prevent Mpox infections.

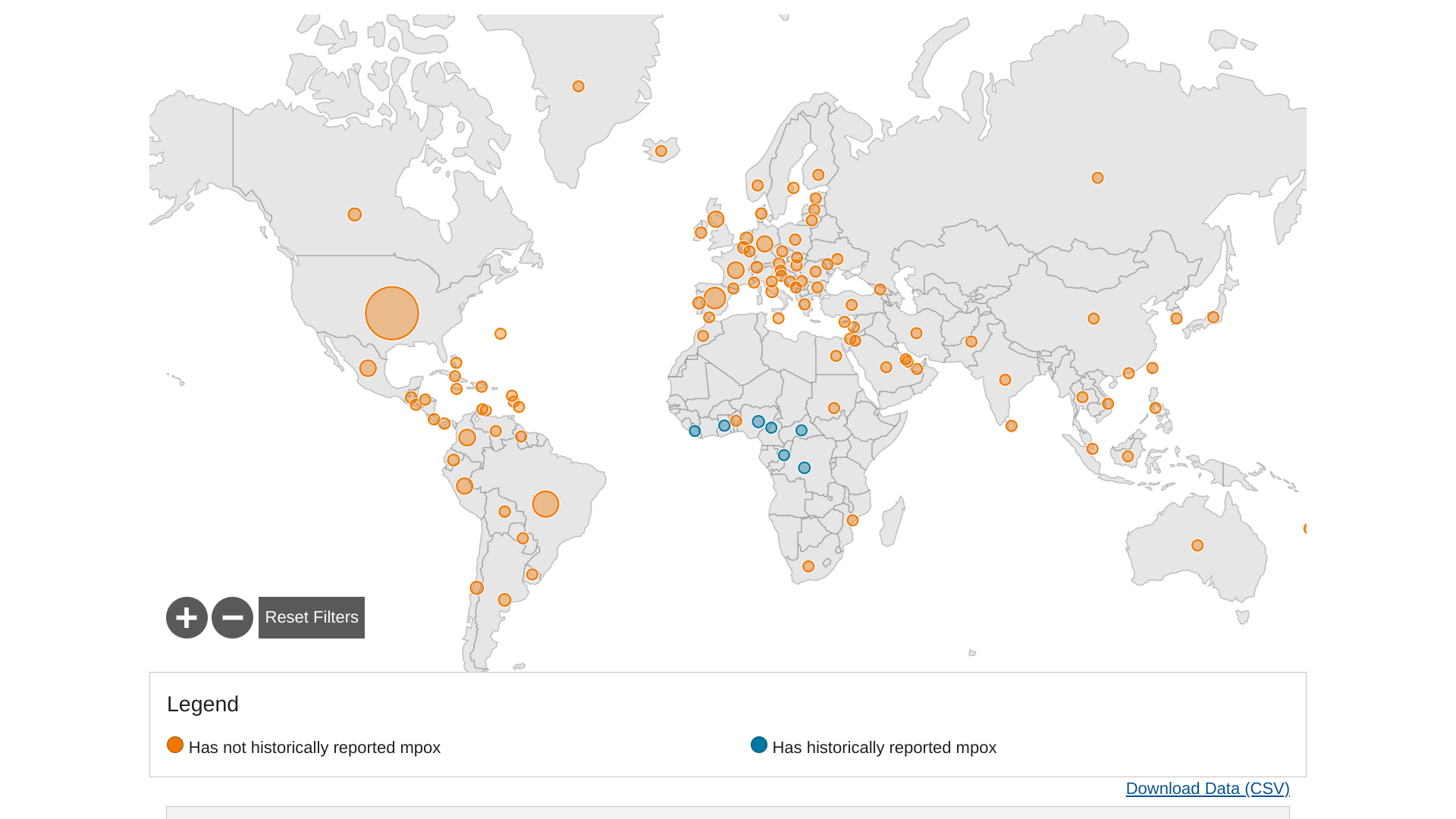
The World Health Organization (WHO) Africa Region recently reported new Mpox cases were reported from the Democratic Republic of the Congo (DRC) and other countries in the past two weeks.
Of reported Mpox cases as of April 30, 2023, 106 new cases were reported from the DRC, two from Nigeria, two from Liberia, and one from Ghana on
Since the start of 2023, DRC has reported 277 laboratory-confirmed cases.
Nineteen (19) Mpox-related fatalities have been reported in the African region since 2022 from Nigeria (9), Ghana (4), Cameroon (3), Central African Republic (1), Mozambique (1), and Sudan (1).
And in the European Region, 17 cases of mpox have been identified from 8 countries and areas over the past four weeks.
Throughout the Mpox outbreak identified in May 2022, 45 countries and areas throughout the European Region have reported cases.
In the U.S., the CDC has reported 30,361 Mpox cases and 42 related fatalities.
As of May 5, 2023, Mpox vaccines, testing, and treatments remain available in most countries.